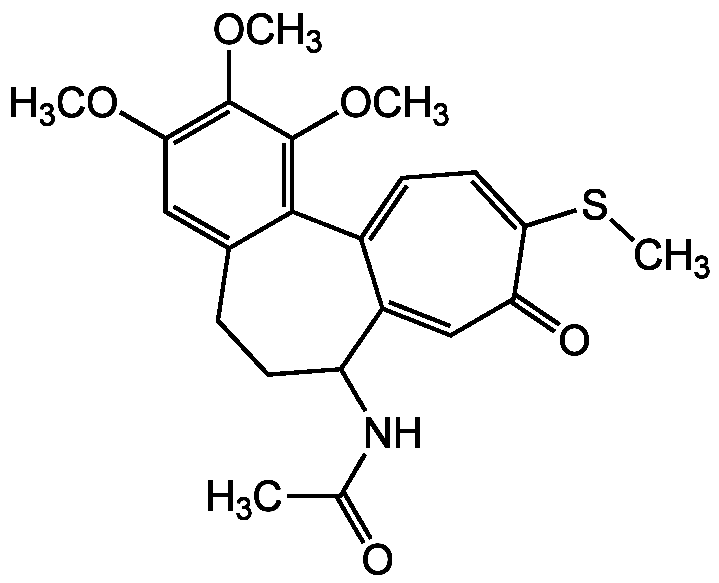
Chemical Structure
Thiocolchicine [2730-71-4]

AG-CN2-0074
Overview
- SupplierAdipoGen Life Sciences
- Product NameThiocolchicine [2730-71-4]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number2730-71-4
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC22H25NO5S
- Molecular Weight415.5
- Scientific DescriptionAntimitotic alkaloid. Tubulin polymerization and microtubule assembly inhibitor. Axonal cytoskeleton modulator. Inhibitor of autophagosome-lysosome fusion. Apoptosis inducer. Thiocolchicine-dimers were shown to be potent topoisomerase I inhibitors. - Chemical. CAS: 2730-71-4. Formula: C22H25NO5S. MW: 415.5. Semisynthetic. Antimitotic alkaloid. Tubulin polymerization and microtubule assembly inhibitor. Axonal cytoskeleton modulator. Inhibitor of autophagosome-lysosome fusion. Apoptosis inducer. Thiocolchicine-dimers were shown to be potent topoisomerase I inhibitors.
- SMILESCOC1=C(OC)C(OC)=C2C(CCC(NC(C)=O)C3=CC(=O)C(SC)=CC=C23)=C1
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 1544
- UNSPSC12352200
References
- Association of thiocolchicine with tubulin: R.M. Chabin & S.B. Hastie; BBRC 161, 544 (1989)
- Effect of tubulin binding and self-association on the near-ultraviolet circular dichroic spectra of colchicine and analogues; R.M. Chabin, et al.; Biochemistry 29, 1869 (1990)
- N-acetylcolchinol O-methyl ether and thiocolchicine, potent analogs of colchicine modified in the C ring. Evaluation of the mechanistic basis for their enhanced biological properties: G.J. Kang, et al.; J. Biol. Chem. 265, 10255 (1990)
- Antitumor agents-CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis: Q. Shi, et al.; Bioorg. Med. Chem. 5, 2277 (1997)
- Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines: R. De Vincenzo, et al.; Anticancer Drug Des. 13, 19 (1998)
- Biological evaluation on different human cancer cell lines of novel colchicine analogs: R. De Vincenzo, et al.; Oncol. Res. 11, 145 (1999)
- Effects of thiocolchicine on axonal cytoskeleton of the rat peroneus nerve: P. Ferri, et al.; Exp. Toxicol. Pathol. 54, 211 (2002)
- Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors: G. Raspaglio, et al.; Biochem. Pharmacol. 69, 113 (2005)
- Inhibitors of tubulin polymerization: synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III: D. Passarella, et al.; Bioorg. Med. Chem. 16, 6269 (2008)
- Synthesis and biological evaluation of novel thiocolchicine-podophyllotoxin conjugates: D. Passarella, et al.; Eur. J. Med. Chem. 45, 219 (2010)
