Chemical Structure
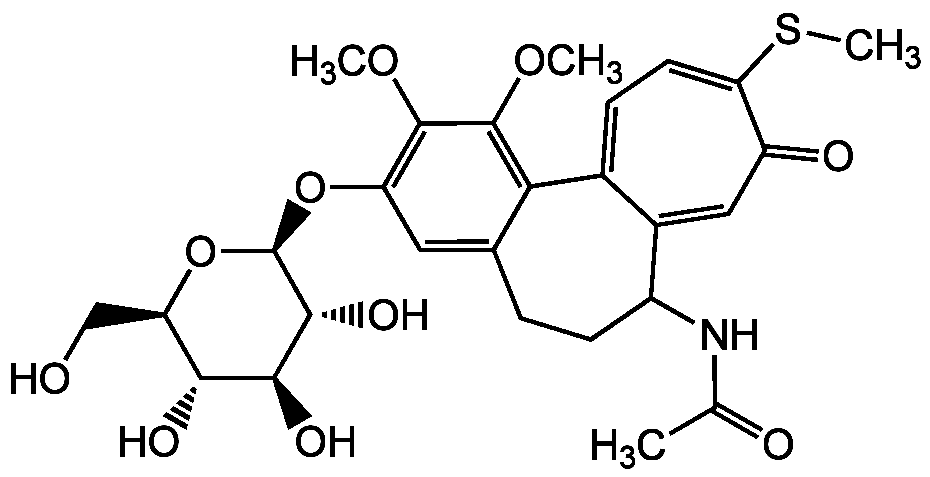
Thiocolchicoside [602-41-5]

AG-CN2-0076
Overview
- SupplierAdipoGen Life Sciences
- Product NameThiocolchicoside [602-41-5]
- Delivery Days Customer10
- CAS Number602-41-5
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC27H33NO10S
- Molecular Weight563.6
- Scientific DescriptionChemical. CAS: 602-41-5. Formula: C27H33NO10S. MW: 563.6. Semisynthetic. Potent competitive gamma-aminobutyric acid type A (GABAA) receptor antagonist and glycine receptor agonist. Weak nicotinic acetylcholine receptor agonist. Muscle relaxant. Anti-inflammatory. Has analgesic properties. Shows strong epileptogenic and convulsant activity. Anticancer compound through inhibition of NF-kappaB and NF-kappaB-regulated gene products Apoptosis inducer. Suppressed osteoclastogenesis induced by RANKL and tumor cells via the NF-kappaB signaling pathway. Therapeutic option for the management of bone metastatic disease. - Potent competitive gamma-aminobutyric acid type A (GABAA) receptor antagonist and glycine receptor agonist. Weak nicotinic acetylcholine receptor agonist. Muscle relaxant. Anti-inflammatory. Has analgesic properties. Shows strong epileptogenic and convulsant activity. Anticancer compound through inhibition of NF-kappaB and NF-kappaB-regulated gene products Apoptosis inducer. Suppressed osteoclastogenesis induced by RANKL and tumor cells via the NF-kappaB signaling pathway. Therapeutic option for the management of bone metastatic disease.
- SMILESCOC1=C(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)C=C2CCC(NC(C)=O)C3=CC(=O)C(SC)=CC=C3C2=C1OC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- Affinity of thiocolchicoside and thiocolchicoside analogues for the postsynaptic GABA receptor site: K. Biziere, et al.; Eur. J. Pharmacol. 75, 167 (1981)
- Review of the toxicology, pharmacodynamics and pharmacokineticss of thiocolchicoside, a GABA-agonist muscle relaxant with anti-inflammatory and analgesic actions: J.M. Janbroers; Acta Ther. 13, 221 (1987)
- Interaction of thiocolchicoside with [3H]strychnine binding sites in rat spinal cord and brainstem: M. Cimino, et al.; Eur. J. Pharmacol. 318, 201 (1996)
- Focal and secondarily generalised convulsive status epilepticus induced by thiocolchicoside in the rat: G. Sechi, et al.; Seizure 12, 508 (2003)
- Multicenter, randomized, double-blinded, placebo-controlled trial of thiocolchicoside in acute low back pain: F. Tüzün, et al.; Joint Bone Spine 70, 356 (2003)
- The muscle relaxant thiocolchicoside is an antagonist of GABAA receptor function in the central nervous system: M. Carta, et al.; Neuropharmacology 51, 805 (2006)
- Thiocolchicoside inhibits the activity of various subtypes of recombinant GABA(A) receptors expressed in Xenopus laevis oocytes: M.P. Mascia, et al.; Eur. J. Pharmacol. 558, 37 (2007)
- Thiocolchicoside exhibits anticancer effects through downregulation of NF-kappaB pathway and its regulated gene products linked to inflammation and cancer: S. Reuter, et al.; Cancer Prev. Res. 3, 1462 (2010)
- Thiocolchicoside suppresses osteoclastogenesis induced by RANKL and cancer cells through inhibition of inflammatory pathways: a new use for an old drug: S. Reuter, et al.; Br. J. Pharmacol. 165, 2127 (2012)
- Thiocolchicoside a semi-synthetic derivative of the Glory Lily: a new weapon to fight metastatic bone resorption? O. Micheau, et al.; Br. J. Pharmacol. 165, 2124 (2012)
