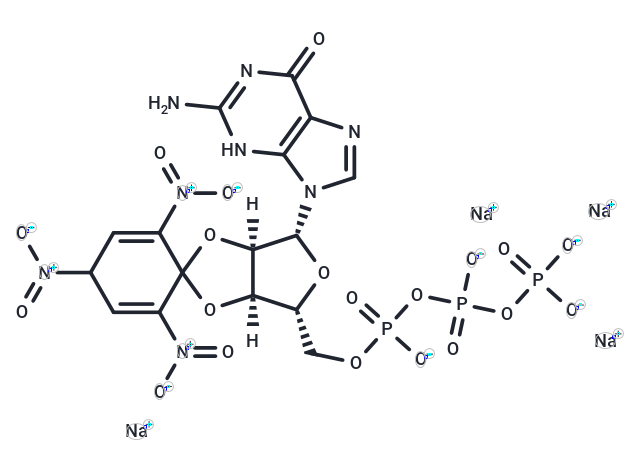
TNP-GTP, a fluorescent derivative of guanosine 5-triphosphate (GTP), essential for protein synthesis and gluconeogenesis, exhibits an emission peak at 552 nm when excited at 410 nm in water. Its fluorescence intensity increases and emission peaks shift to 544 nm and 532 nm in 40% and 80% N,N-dimethylformamide, respectively, due to the solvents lower polarity compared to water. As a potent inhibitor of glutamate dehydrogenase (Ki = 2.7 microM), TNP-GTP enhances fluorescence and shifts its emission to 545 nm upon binding to this enzyme, a process reversible by GTP addition. Additionally, it serves as an antagonist to purinergic P2X2 and P2X2/3 receptors (IC50s = 0.4 and 1.2 nM, respectively) and selectively inhibits rat soluble guanylyl cyclase (sGC; Ki = 11 nM) over bovine liver glutamate dehydrogenase (GDH; Ki = 2.7 microM) and the calmodulin-dependent B. pertussis adenylyl cyclase (AC) toxin, with inhibition constants of 20 microM and 320 microM in the presence of manganese and magnesium, respectively.