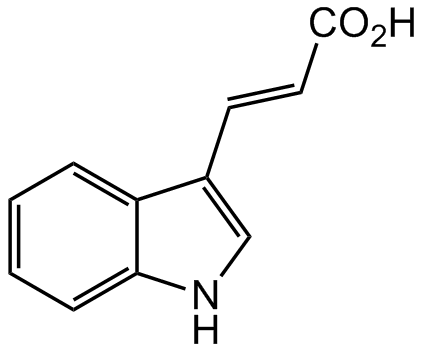
Chemical Structure
trans-Indole-3-acrylic acid [29953-71-7]

AG-CR1-3677
Overview
- SupplierAdipoGen Life Sciences
- Product Nametrans-Indole-3-acrylic acid [29953-71-7]
- Delivery Days Customer10
- CAS Number29953-71-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC11H9NO2
- Molecular Weight187.2
- Scientific DescriptionChemical. CAS: 29953-71-7. Formula: C11H9NO2. MW: 187.2. Synthetic. Inflammation suppressor. Tryptophan metabolite produced by human microbiota (intestinal commensale Peptostreptococcus sp). Involved in keeping the intestinal barrier intact and works as anti-inflammatory molecule. Patients with Inflammatory bowel Disease (IBD) have an altered microbiota, with less Peptostreptococcus sp., and therefore reduced production of trans-indole-3-acrylic acid. Chromophoric L-Trp analog used to probe the allosteric properties of the internal aldimine of tryptophan synthas. Also shown to be a moderate inhibitor of tryptophan synthase, trypothan-2,3-dioxygenase, indoleamine-2,3-dioxygenase, L-dopachrome isomerase and xanthine oxidase. Reagent used as a matrix for MALDI-TOF mass spectroscopy in order to characterize and analyze polyphenols and synthetic polymers. Used as heterocyclic building block or intermediate for the synthesis of indolyl acrylamide-derived inhibitors, pharmaceuticals or agrochemicals. - Inflammation suppressor. Tryptophan metabolite produced by human microbiota (intestinal commensale Peptostreptococcus sp.). Involved in keeping the intestinal barrier intact and works as anti-inflammatory molecule. Patients with Inflammatory Bowel Disease (IBD) have an altered microbiota, with less Peptostreptococcus sp. and therefore reduced production of trans-indole-3-acrylic acid. Chromophoric L-Trp analog used to probe the allosteric properties of the internal aldimine of tryptophan synthase. Also shown to be a moderate inhibitor of tryptophan synthase, trypothan-2,3-dioxygenase (TDO), indoleamine-2,3-dioxygenase (IDO), L-dopachrome isomerase and xanthine oxidase. Reagent used as a matrix for MALDI-TOF mass spectroscopy in order to characterize and analyze polyphenols and synthetic polymers. Used as heterocyclic building block or intermediate for the synthesis of indolyl acrylamide-derived inhibitors, pharmaceuticals or agrochemicals.
- SMILESO=C(/C=C/C1=CNC2=CC=CC=C21)O
- Storage Instruction2°C to 8°C
- UNSPSC12352200
References
- Inhibition of tryptophan synthetase by indoleacrylic acid: W.H. Matchett; J. Bacteriol. 110, 146 (1972)
- Stereospecificity of hepatic L-tryptophan 2,3-dioxygenase: Y. Watanabe, et al.; Biochem. J. 189, 393 (1980)
- Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by beta-carboline and indole derivatives: N. Eguchi; Arch. Biochem. Biophys. 232, 602 (1984)
- The structural basis for the interaction between L-tryptophan and the Escherichia coli trp aporepressor: R.Q. Marmorstein, et al.; J. Biol. Chem. 262, 4922 (1987)
- Interaction of Indoleacrylic Acid with Trp aporepressor from Escherichia coli: D. Hu & M.R. Eftink; Arch. Biochem. Biophys. 305, 588 (1993)
- Photosensizied inactivation of infectious DNA by urocanic acid, indoleacrylic acid and rhodiuk complexes: T. Mohammad, et al.; Photochem. Photobiol. 59, 189 (1994)
- Desorption behavior and distributions of fluorinated polymers in MALDI and electrospray ionization mass spectrometry: L. Latourte, et al.; Anal. Chem. 69, 2742 (1997)
- Substrate specificity for isomerase activity of macrophage migration inhibitory factor and its inhibition by indole derivatives: M. Suzuki, et al.; J. Biochem. 122, 1040 (1997)
- Where does indolylacrylic acid come from?: E. Marklova; Amino Acids 17, 401 (1999)
- Novel allosteric effectors of the tryptophan synthase K2L2 complex identifed by computer-assisted molecular modelling: A. Marabotti, et al.; Biochim. Biophys. Acta 1476, 287 (2000)
