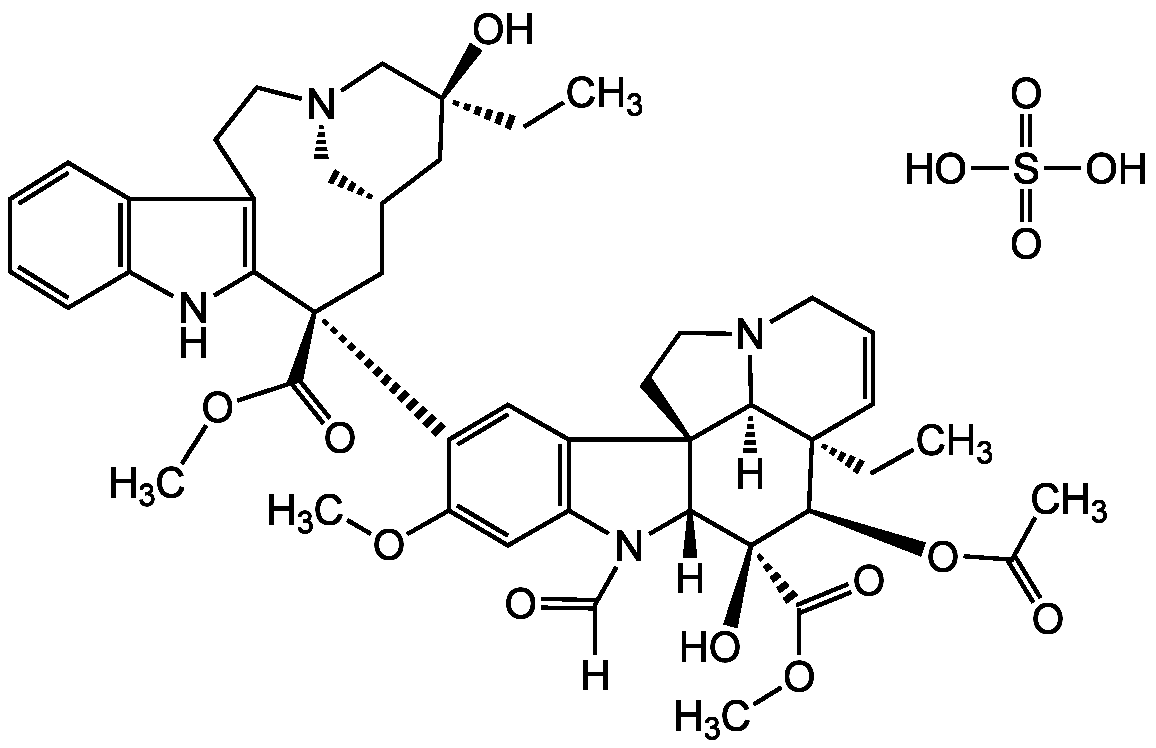
Chemical Structure
Vincristine . sulfate [2068-78-2]

AG-CN2-0446
CAS Number2068-78-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight825.0 . 98.1
Overview
- SupplierAdipoGen Life Sciences
- Product NameVincristine . sulfate [2068-78-2]
- Delivery Days Customer10
- ADR Class6.1
- CAS Number2068-78-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC46H56N4O10 . H2SO4
- Molecular Weight825.0 . 98.1
- Scientific DescriptionChemical. CAS: 2068-78-2. Formula: C46H56N4O10 . H2SO4. MW: 825.0 . 98.1. Synthetic. Originally isolated from Vinca rosea. Immunosuppressant. Anticancer compound. Chemotherapeutic agent. Depolymerizes microtubules and irreversibly blocks binding of tubulin to microtubule proteins. Apoptosis inducer and cell cycle inhibitor in G2/M phase. Autophagy inhibitor. Inhibits microtubule-mediated fusion of autophagosome with lysosme. Inducer of IL-1beta production. Activates alone and synergistically together with doxorubicin the NLRP3-inflammasome mediated processing of IL-1beta. Monoamine oxidase (MAO) inhibitor. - Immunosuppressant. Anticancer compound. Chemotherapeutic agent. Depolymerizes microtubules and irreversibly blocks binding of tubulin to microtubule proteins. Apoptosis inducer and cell cycle inhibitor in G2/M phase. Autophagy inhibitor. Inhibits microtubule-mediated fusion of autophagosome with lysosme. Inducer of IL-1beta production. Activates alone and synergistically together with doxorubicin the NLRP3-inflammasome mediated processing of IL-1beta. Monoamine oxidase (MAO) inhibitor.
- SMILESOS(O)(=O)=O.[H]C(=O)N1C2=C(C=C(C(OC)=C2)[C@]2(C[C@@H]3C[N@@](C[C@](O)(CC)C3)CCC3=C2NC2=C3C=CC=C2)C(=O)OC)[C@@]23CCN4CC=C[C@@](CC)([C@@H](OC(C)=O)[C@](O)(C(=O)OC)[C@]12[H])[C@@]34[H]
- Storage Instruction2°C to 8°C,RT
- UN NumberUN 2811
- UNSPSC12352200
References
- Suppression of immune response by Vincristine and Vinblastine: A.C. Aisenberg; Nature 200, 484 (1963)
- Vinblastine and vincristine are inhibitors of monoamine oxidase B: J.K. Son, et al.; J. Med. Chem. 33, 1845 (1990)
- Cell death induced by vincristine in the intestinal crypts of mice and in a human Burkitt's lymphoma cell line: B.V. Harmon, et al.; Cell Prolif. 25, 523 (1992)
- Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine: S. Lobert, et al.; Biochemistry 35, 6806 (1996)
- Antimitotic natural products and their interactions with tubulin: E. Hamel; Med. Res. Rev. 16, 207 (1996)
- Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death: M.V. Blagosklonny, et al.; Cancer Res. 57, 130 (1997)
- Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle: A. Jordan, et al.; Med. Res. Rev. 18, 259 (1998) (Review)
- The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review: L.G. Wang; Cancer Chemother. Pharmacol. 44, 355 (1999) (Review)
- Vincristine-induced apoptosis in vivo in peripheral blood mononuclear cells of children with acute lymphoblastic leukaemia (ALL): E. Groninger, et al.; Br. J. Haematol. 111, 875 (2000)
- Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1: Z. Zhan, et al.; Autophagy 8, 109 (2012)
