Chemical Structure
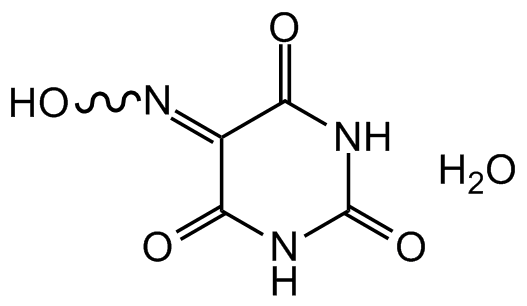
Violuric acid monohydrate [26351-19-9]
CDX-V0006
CAS Number26351-19-9
Product group Chemicals
Estimated Purity>97%
Molecular Weight175.1
Overview
- SupplierChemodex
- Product NameVioluric acid monohydrate [26351-19-9]
- Delivery Days Customer10
- CAS Number26351-19-9
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationWarning
- Molecular FormulaC4H3N3O4 . H2O
- Molecular Weight175.1
- Scientific DescriptionChemical. CAS: 26351-19-9. Formula: C4H3N3O4 . H2O. Molecular Weight: 175.1. Violuric acid has been used as an analytical reagent for spectrophotometric detection of cations, chromatographic separation and for cation oxidation (.e.g Co2+, Cu2+, Fe2+, etc). Violuric acid forms solid complexes (chelates) with many different cations and has attracted attention because simple salts of the violurate anion are deeply colored. The colours depend on the hydrate and the ratio of acid to cation, pH, temperature. Violuric acid is a redox mediator, and is used as reporter assay for assessing redox potentials during protein engineering. Violuric acid is effectively oxidized only by high-redox potential laccases (HRPLs) due to its high-redox potential. New colorimetric screening assays for the directed evolution of fungal laccases to improve the conversion of plant biomass have been described. Violuric acid has also been used for the optimization of reactive textile dyes degradation by the laccase-mediator system and as a mediator of lignin degradation by fungal laccase. Laccase is the generic name given to a family of multicopper oxidases that are capable of oxidizing several different substrates with the concomitant reduction of dioxygen to water. Although these enzymes exhibit specific affinity for oxygen as their electron acceptor, their specificity towards their reducing substrates is rather low. Laccases catalyze the removal of a hydrogen atom from the hydroxyl group of methoxy-substituted monophenols, ortho- and para-diphenols; they also oxidize other substrates such as aromatic amines, syringaldazine and non-phenolic compounds to form free radicals. The capability of laccase to degrade chromophores such as triarylmethane, indigoid, azo and anthraquinoid suggests that it offers potential application in textile dye bleaching processes. However, these processes have been hindered due to unfavourable kinetics between the enzyme and the dye; the use of small molecules that are capable of acting as electron transfer mediators between the enzyme and the dye has been regarded as a feasible solution to this particular drawback. When mediators are incorporated in laccase-assisted processes, electron transfer from the mediator to the enzyme is followed by electron donations from the target molecule to the oxidized mediator, which causes the regeneration of the mediator. In this context, compounds other than laccase substrates capable of undergoing indirect catalytic oxidation reactions can be treated with a laccase-mediator system (LMS). Violuric acid is the most effective redox mediator for laccase oxidation reactions. Violuric acid has also been used s a pharmaceutical intermediate. - Violuric acid has been used as an analytical reagent for spectrophotometric detection of cations, chromatographic separation and for cation oxidation (.e.g Co2+, Cu2+, Fe2+, etc). Violuric acid forms solid complexes (chelates) with many different cations and has attracted attention because simple salts of the violurate anion are deeply colored. The colours depend on the hydrate and the ratio of acid to cation, pH, temperature. Violuric acid is a redox mediator, and is used as reporter assay for assessing redox potentials during protein engineering. Violuric acid is effectively oxidized only by high-redox potential laccases (HRPLs) due to its high-redox potential. New colorimetric screening assays for the directed evolution of fungal laccases to improve the conversion of plant biomass have been described. Violuric acid has also been used for the optimization of reactive textile dyes degradation by the laccase-mediator system and as a mediator of lignin degradation by fungal laccase. Laccase is the generic name given to a family of multicopper oxidases that are capable of oxidizing several different substrates with the concomitant reduction of dioxygen to water. Although these enzymes exhibit specific affinity for oxygen as their electron acceptor, their specificity towards their reducing substrates is rather low. Laccases catalyze the removal of a hydrogen atom from the hydroxyl group of methoxy-substituted monophenols, ortho- and para-diphenols; they also oxidize other substrates such as aromatic amines, syringaldazine and non-phenolic compounds to form free radicals. The capability of laccase to degrade chromophores such as triarylmethane, indigoid, azo and anthraquinoid suggests that it offers potential application in textile dye bleaching processes. However, these processes have been hindered due to unfavourable kinetics between the enzyme and the dye; the use of small molecules that are capable of acting as electron transfer mediators between the enzyme and the dye has been regarded as a feasible solution to this particular drawback. When mediators are incorporated in laccase-assisted processes, electron transfer from the mediator to the enzyme is followed by electron donations from the target molecule to the oxidized mediator, which causes the regeneration of the mediator. In this context, compounds other than laccase substrates capable of undergoing indirect catalytic oxidation reactions can be treated with a laccase-mediator system (LMS). Violuric acid is the most effective redox mediator for laccase oxidation reactions. Violuric acid has also been used s a pharmaceutical intermediate.
- SMILESO=C(/C(C(N1)=O)=N/O)NC1=O.O
- Storage InstructionRT
- UNSPSC12352200