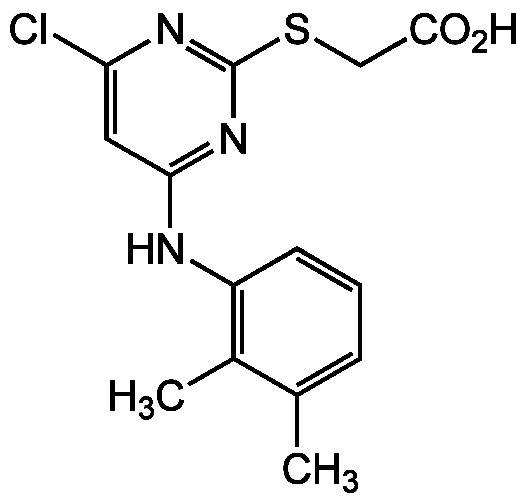
Chemical Structure
WY-14643 [50892-23-4]

AG-CR1-3566
Overview
- SupplierAdipoGen Life Sciences
- Product NameWY-14643 [50892-23-4]
- Delivery Days Customer10
- CAS Number50892-23-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger
- Molecular FormulaC14H14ClN3O2S
- Molecular Weight323.8
- Scientific DescriptionChemical. CAS: 50892-23-4. Formula: C14H14ClN3O2S. MW: 323.8. Potent peroxisome proliferator-activated receptor (PPARalpha) activator. Activates also PPARgamma but not PPARdelta. Potent anti-hypercholesterolemic agent. Hypolipidemic compound. Lipogenesis inducer. Tumor promoter. Causes increased cell proliferation and decreased apoptosis. Anti-inflammatory. Inhibits NF-kappaB transcriptional activity and decreases the inflammatory response by reducing the production of inflammatory cytokines (TNF-alpha, IL1beta). Reduces oxidative stress. Increases fatty acid oxidation. Directly affects insulin signaling. Increases glucose uptake. Reviews. - Potent peroxisome proliferator-activated receptor (PPARalpha) activator [1, 2, 7, 9]. Activates also PPARgamma [4, 7] but not PPARdelta [8]. Potent anti-hypercholesterolemic agent [1]. Hypolipidemic compound. Lipogenesis inducer [2, 10]. Tumor promoter [2, 3, 5, 15]. Causes increased cell proliferation and decreased apoptosis [5]. Anti-inflammatory. Inhibits NF-kappaB transcriptional activity and decreases the inflammatory response by reducing the production of inflammatory cytokines (TNF-alpha, IL1beta). Reduces oxidative stress [6, 7, 12, 13]. Increases fatty acid oxidation [11]. Directly affects insulin signaling. Increases glucose uptake [12]. Enhancer of ethanol metabolism. Reviews [5, 11, 14].
- SMILESCC1=CC=CC(NC2=NC(SCC(O)=O)=NC(Cl)=C2)=C1C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
References
- A potent antihypercholesterolemic agent: (4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio) acetic acid (Wy-14643): A.A. Santilli, et al.; Experientia 30, 1110 (1974)
- The hepatic effects of hypolipidemic drugs (clofibrate, nafenopin, tibric acid, and Wy-14,643) on hepatic peroxisomes and peroxisome-associated enzymes: D.E. Moody & J.K. Reddy; Am. J. Pathol. 90, 435 (1978)
- Peroxisome proliferation and hepatocarcinogenesis: M.S. Rao & J.K. Reddy; Carcinogenesis 8, 631 (1987)
- Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids: A. Schmidt, et al.; Mol. Endocrinol. 6, 1634 (1992)
- Non-genotoxic hepatocarcinogenesis: suppression of apoptosis by peroxisome proliferators: R.A. Roberts; Ann. N. Y. Acad. Sci. 804, 588 (1996) (Review)
- The PPARalpha-leukotriene B4 pathway to inflammation control: P.R. Devchand, et al.; Nature 384, 39 (1996)
- Peroxisome proliferator-activated receptors a and g are activated by indomethacin and other non-steroidal anti-inflammatory drugs: J.M. Lehmann, et al.; J. Biol. Chem. 272, 3406 (1997)
- Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta: B.M. Forman, et al.; PNAS 94, 4312 (1997)
- Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators: B. Staels, et al.; Nature 393, 790 (1998)
- Influence of peroxisome proliferator-activated receptor alpha agonists on the intracellular turnover and secretion of apolipoprotein (Apo) B-100 and ApoB-48: D. Lindén, et al.; J. Biol. Chem. 277, 23044 (2002)
