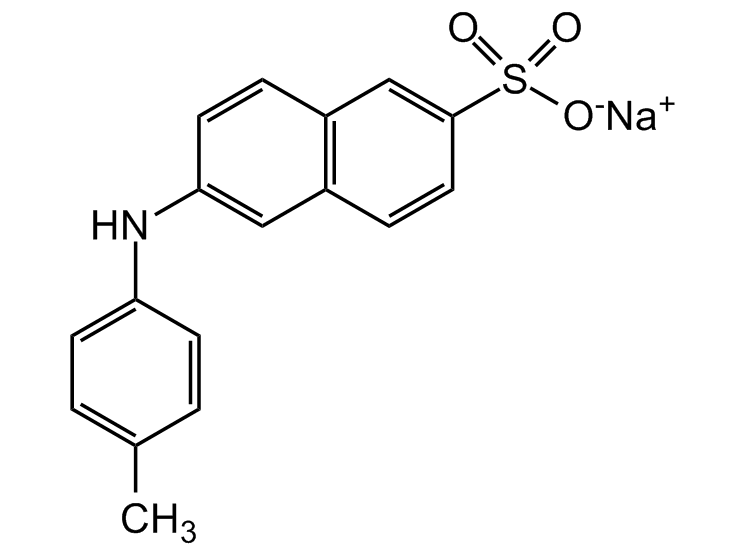
Chemical Structure
6-(p-Toluidino)-2-naphthalenesulfonic acid sodium salt [53313-85-2] [53313-85-2]
CDX-T0071
CAS Number53313-85-2
Product group Chemicals
Estimated Purity0.99
Molecular Weight335.35
Overview
- SupplierChemodex
- Product Name6-(p-Toluidino)-2-naphthalenesulfonic acid sodium salt [53313-85-2] [53313-85-2]
- Delivery Days Customer10
- CAS Number53313-85-2
- CertificationResearch Use Only
- Estimated Purity0.99
- Hazard InformationWarning
- Molecular FormulaC17H14NNaO3S
- Molecular Weight335.35
- Scientific Description6-(p-Toluidino)-2-naphthalenesulfonic acid (TNS) is a widely recognized aminonaphthalene-based fluorescent probe characterized for its solvatochromic effect. TNS is found to be almost fluorescence silent in aqueous medium whereas shows a high quantum yield in organic solvents with a hypsochromic shift, as the polarity of the medium decreases. This property has been exploited in biological studies to probe the micellation properties of surfactants, membrane fluidity, hydrophobic surfaces on proteins, conformational changes upon ligand binding, ligand binding events, protein-surfactant interactions, multimeric protein assembly or aggregation, fibril formation and others. TNS has been used in many studies as environmentally sensitive fluorescent probe for the conformational state of proteins. TNS fluorescence enhancement upon binding to unfolded states of proteins might be due to the hydrophobic environment and reduced solvent accessibility rather than the binding ability and specific orientation of TNS in the bound state. Studies suggest that TNS forms aggregates in water whereas in non-aqueous solvents the order of aggregates is lower which might result in an enhancement of its fluorescence intensity. Further, TNS preferably interacts with basic and aromatic amino acid residues of the proteins. TNS is a well characterized fluorescent probe of protein structure that fluoresces in nonpolar environments but is quenched and red-shifted in solution. Spectral data: lambdaex 320nm; lambdaem ~440nm. - Chemical. CAS: 53313-85-2. Formula: C17H14NNaO3S. Molecular Weight: 335.35. 6-(p-Toluidino)-2-naphthalenesulfonic acid (TNS) is a widely recognized aminonaphthalene-based fluorescent probe characterized for its solvatochromic effect. TNS is found to be almost fluorescence silent in aqueous medium whereas shows a high quantum yield in organic solvents with a hypsochromic shift, as the polarity of the medium decreases. This property has been exploited in biological studies to probe the micellation properties of surfactants, membrane fluidity, hydrophobic surfaces on proteins, conformational changes upon ligand binding, ligand binding events, protein-surfactant interactions, multimeric protein assembly or aggregation, fibril formation and others. TNS has been used in many studies as environmentally sensitive fluorescent probe for the conformational state of proteins. TNS fluorescence enhancement upon binding to unfolded states of proteins might be due to the hydrophobic environment and reduced solvent accessibility rather than the binding ability and specific orientation of TNS in the bound state. Studies suggest that TNS forms aggregates in water whereas in non-aqueous solvents the order of aggregates is lower which might result in an enhancement of its fluorescence intensity. Further, TNS preferably interacts with basic and aromatic amino acid residues of the proteins. TNS is a well characterized fluorescent probe of protein structure that fluoresces in nonpolar environments but is quenched and red-shifted in solution. Spectral data: lambdaex 320nm; lambdaem ~440nm.
- SMILESCC(C=C1)=CC=C1NC2=CC3=C(C=C2)C=C(S(=O)([O-])=O)C=C3.[Na+]
- Storage Instruction2°C to 8°C,RT
- UNSPSC12162000
