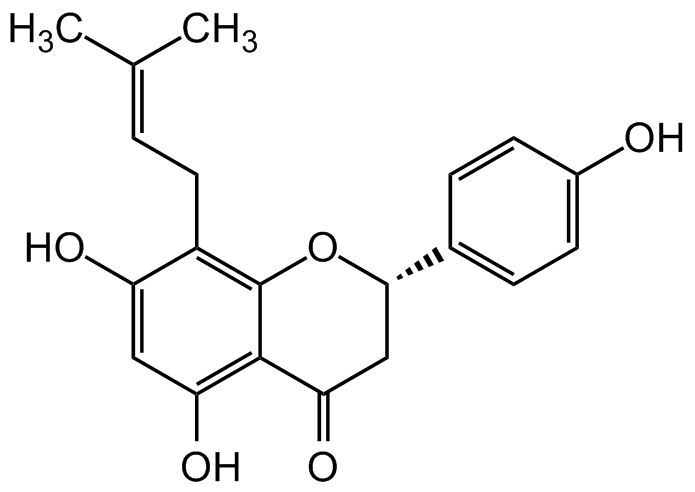
Chemical Structure
8-Prenylnaringenin [53846-50-7] [53846-50-7]
AG-CN2-0525
CAS Number53846-50-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight340.4
Overview
- SupplierAdipoGen Life Sciences
- Product Name8-Prenylnaringenin [53846-50-7] [53846-50-7]
- Delivery Days Customer10
- CAS Number53846-50-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC20H20O5
- Molecular Weight340.4
- Scientific DescriptionChemical. CAS: 53846-50-7. Formula: C20H20O5. MW: 340.4. Prenylflavonoid non-steroidal phytoestrogen that mimicks and/or modulates endogenous estrogens via estrogen receptor binding. Inhibits both isoforms of the human estrogen receptor (ER; IC50s = 57nM and 68nM for ERalpha and ERbeta, respectively). Acts as a full agonist of ERalpha. Cancer chemopreventive agent. Shown to decrease ROS and increase the oxidative phosphorylation system (OXPHOS). Anticancer agent with antiangiogenic and antiproliferative properties. Shown to inhibit cell cycle progression, induce apoptosis in MCF-7 cells and induce autophagy in prostate cancer cell lines. Potent aromatase inhibitor (IC50=65nM) and cellular histone deacetylases (HDACs) inhibitor. Anti-diabetic agent. Activates AMPK signaling consequently suppressing lipogenesis. Shown to prevent body weight gain, improve insulin resistance and glucose tolerance. Potent uncompetitive tight-binding inhibitor of human aldose reductase AKR1B1 (Ki=0.71microM) and of human AKR1B10 (Ki=1.95microM), both pharmacological targets in cancer therapy (AKR1B10) and in the treatment of diabetic complications (AKR1B1). Anti-osteoporetic agent. Effective in vivo, suppressing loss of bone mineral density. Anti-inflammatory and vascularprotective compound. Potential lead structure for potential new therapeutic agents. - Prenylflavonoid non-steroidal phytoestrogen that mimicks and/or modulates endogenous estrogens via estrogen receptor binding. Inhibits both isoforms of the human estrogen receptor (ER; IC50s = 57nM and 68nM for ERalpha and ERbeta, respectively). Acts as a full agonist of ERalpha. Cancer chemopreventive agent. Shown to decrease ROS and increase the oxidative phosphorylation system (OXPHOS). Anticancer agent with antiangiogenic and antiproliferative properties. Shown to inhibit cell cycle progression, induce apoptosis in MCF-7 cells and induce autophagy in prostate cancer cell lines. Potent aromatase inhibitor (IC50=65nM) and cellular histone deacetylases (HDACs) inhibitor. Anti-diabetic agent. Activates AMPK signaling consequently suppressing lipogenesis. Shown to prevent body weight gain, improve insulin resistance and glucose tolerance. Potent uncompetitive tight-binding inhibitor of human aldose reductase AKR1B1 (Ki=0.71microM) and of human AKR1B10 (Ki=1.95microM), both pharmacological targets in cancer therapy (AKR1B10) and in the treatment of diabetic complications (AKR1B1). Anti-osteoporetic agent. Effective in vivo, suppressing loss of bone mineral density. Anti-inflammatory and vascularprotective compound. Potential lead structure for potential new therapeutic agents.
- SMILESOC1=C(C(C[C@@H](C2=CC=C(O)C=C2)O3)=O)C3=C(C/C=C(C)/C)C(O)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![8- Prenylnaringenin [53846-50-7] [53846-50-7]](https://www.targetmol.com/group3/M00/35/89/CgoaEWayI8yECv9dAAAAAJAUaPg449.png)