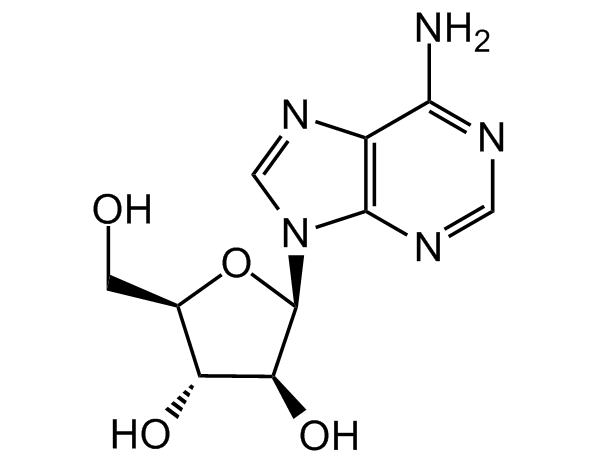
Chemical Structure
Adenine 9-beta-D-arabinofuranoside [5536-17-4] [5536-17-4]
CDX-A0279
CAS Number5536-17-4
Product group Chemicals
Estimated Purity>99%
Molecular Weight267.24
Overview
- SupplierChemodex
- Product NameAdenine 9-beta-D-arabinofuranoside [5536-17-4] [5536-17-4]
- Delivery Days Customer2
- CAS Number5536-17-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC10H13N5O4
- Molecular Weight267.24
- Scientific DescriptionAdenine 9-beta-D-arabinofuranoside is an analog of the nucleoside adenosine that has antiviral properties. It acts as a prodrug that, once phosphorylated by cellular enzymes, acts as both substrate and inhibitor of DNA polymerase. It has been shown to be effective against H. simplex, V. zoster and Epstein-Barr viruses. It is a potent inhibitor of AMP-activated protein kinase (AMPK) in liver, muscle and cardiac cells H9c2. It is also a neurotransmitter that acts as the preferred endogenous agonist at all adenosine receptor subtypes and showed anti-neoplastic activities. It has been shown to have inhibitory activity against SARS-CoV-2-ACE2 binding. - Chemical. CAS: 5536-17-4. Formula: C10H13N5O4. MW: 267.24. Adenine 9-beta-D-arabinofuranoside is an analog of the nucleoside adenosine that has antiviral properties. It acts as a prodrug that, once phosphorylated by cellular enzymes, acts as both substrate and inhibitor of DNA polymerase. It has been shown to be effective against H. simplex, V. zoster and Epstein-Barr viruses. It is a potent inhibitor of AMP-activated protein kinase (AMPK) in liver, muscle and cardiac cells H9c2. It is also a neurotransmitter that acts as the preferred endogenous agonist at all adenosine receptor subtypes and showed anti-neoplastic activities. It has been shown to have inhibitory activity against SARS-CoV-2-ACE2 binding.
- SMILESNC1=NC=NC2=C1N=CN2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O
- Storage Instruction-20°C
- UNSPSC12352200


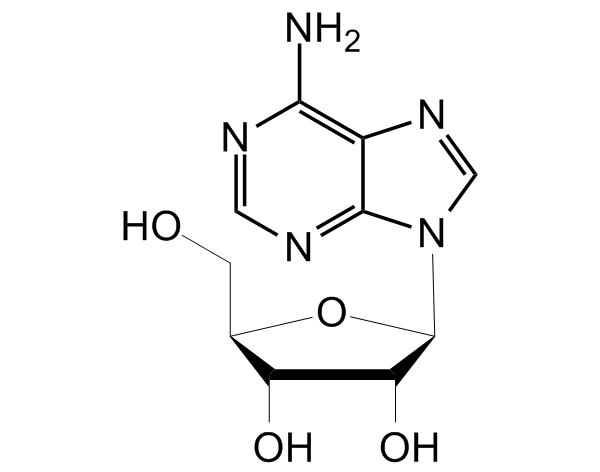
![Vidarabine [5536-17-4] [5536-17-4]](https://www.targetmol.com/group3/M00/02/A8/CgoaEGY7OfmEQuVZAAAAAE1dFos492.png)