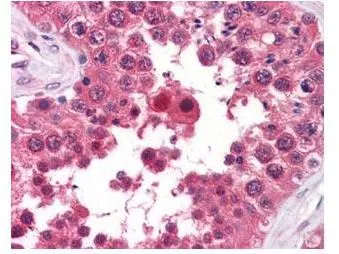
GeneTex's anti-AHA1 monoclonal antibody was used at a 5-10 μg/mL to detect AHA1 in the seminiferous tubule of human testis (40X) showing moderate staining. Leydig cells showed faint to moderate staining. Expression of AHA1 is reported in many epithelial and lymphatic tissues, with cytoplasmic localization. This antibody showed moderate cytoplasmic staining of a variety of epithelial tissues and lymphoid organs such as spleen and tonsil with minimal background staining. The image shows the localization of the antibody as the precipitated red signal, with a hematoxylin purple nuclear counterstain. Tissue was formalin-fixed and paraffin embedded.
AHA-1 antibody [25F2.D9]
GTX48775
ApplicationsWestern Blot, ELISA, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
Product group Antibodies
ReactivityHuman, Mouse
TargetAhsa1
Overview
- SupplierGeneTex
- Product NameAHA-1 antibody [25F2.D9]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:1000. IHC-P: 5-10 microg/mL. ELISA: 1:20000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsWestern Blot, ELISA, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID25F2.D9
- Concentration1 mg/ml
- ConjugateUnconjugated
- Gene ID217737
- Target nameAhsa1
- Target descriptionAHA1, activator of heat shock protein ATPase 1
- Target synonymsp38, activator of 90 kDa heat shock protein ATPase homolog 1, AHA1, activator of heat shock 90kDa protein ATPase homolog 1, AHA1, activator of heat shock protein ATPase homolog 1
- HostRat
- IsotypeIgG2a
- Protein IDQ8BK64
- Protein NameActivator of 90 kDa heat shock protein ATPase homolog 1
- Scientific DescriptionActivator of Hsp90 ATPase (AHA1) stimulates the inherent ATPase cycle of Hsp90, which is essential for its chaperone activity in vivo. The activation and/or stability of many of the key regulatory and signaling proteins of the eukaryotic cell depend on their interaction with the Hsp90 molecular chaperone. Hsp90 is assisted and regulated by co-chaperones that participate in an ordered series both to assist client-protein recruitment or release and to modulate progress through the ATPase coupled chaperone cycle. Structural analysis and mutagenesis show that binding of the N-terminal domain of AHA1 to Hsp90 promotes a conformational switch in the middle-segment catalytic loop (aa 370-390) of Hsp90 that exposes the catalytic Arg380 and enables its interaction with ATP in the N-terminal nucleotide-binding domain of the chaperone. Recent studies show that AHA1 modulates Hsp90-dependent stability of the folding of the cystic fibrosis transmembrane conductance regulator (CFTR) in the endoplasmic reticulum (ER). Down-regulation of AHA1 rescues misfolding of CFTR in cystic fibrosis.
- ReactivityHuman, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161

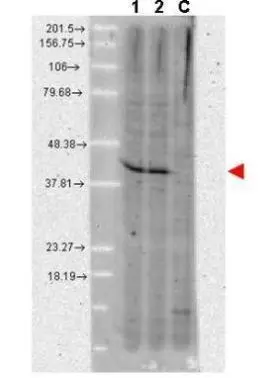
![WB analysis of various samples using GTX48775 AHA-1 antibody [25F2.D9]. Lane 1 : A431 whole cell lysate Lane 2 : MCF-7 whole cell lysate Lane C : Without primary antibody Dilution : 1:1000 WB analysis of various samples using GTX48775 AHA-1 antibody [25F2.D9]. Lane 1 : A431 whole cell lysate Lane 2 : MCF-7 whole cell lysate Lane C : Without primary antibody Dilution : 1:1000](https://www.genetex.com/upload/website/prouct_img/normal/GTX48775/GTX48775_20240423_WB_302_24042320_368.webp)