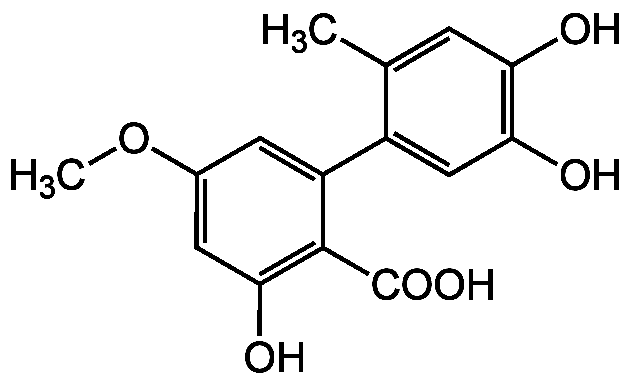
Chemical Structure
Altenusin [31186-12-6] [31186-12-6]
AG-CN2-0143
CAS Number31186-12-6
Product group Chemicals
Estimated Purity>97%
Molecular Weight290.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameAltenusin [31186-12-6] [31186-12-6]
- Delivery Days Customer10
- CAS Number31186-12-6
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationWarning
- Molecular FormulaC15H14O6
- Molecular Weight290.3
- Scientific DescriptionAntibiotic [7]. Antifungal penicillide [8]. Non-competitive, specific neutral sphingomyelinase (N-SMase) inhibitor [2]. Strong pp60c-Src inhibitor [3]. Inhibits cFMS receptor tyrosine kinase (CSF-1/m-CSF receptor tyrosine kinase) which is implicated in cancer and bone diseases [3]. Myosin light chain kinase inhibitor [1]. Exhibits anti-HIV-1 integrase activity [4]. Cytotoxic activity against mouse lymphoma cells [6]. Anti-protozoan, trypanothione reductase (TR) inhibitor [5]. Phytotoxin [9]. - Chemical. CAS: 31186-12-6. Formula: C15H14O6. MW: 290.3. Isolated from Penicillium sp. Antibiotic. Antifungal penicillide. Non-competitive, specific neutral sphingomyelinase (N-SMase) inhibitor. Strong pp60c-Src inhibitor. Inhibits cFMS receptor tyrosine kinase (CSF-1/m-CSF receptor tyrosine kinase) which is implicated in cancer and bone diseases. Myosin light chain kinase inhibitor. Exhibits anti-HIV-1 integrase activity. Cytotoxic activity against mouse lymphoma cells. Anti-protozoan, trypanothione reductase (TR) inhibitor. Phytotoxin.
- SMILESCOC1=CC(O)=C(C(O)=O)C(=C1)C1=CC(O)=C(O)C=C1C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Altenusin [31186-12-6] [31186-12-6]](https://www.targetmol.com/group3/M00/02/59/CgoaEWY7KtmEKA7DAAAAAKGM09g344.png)