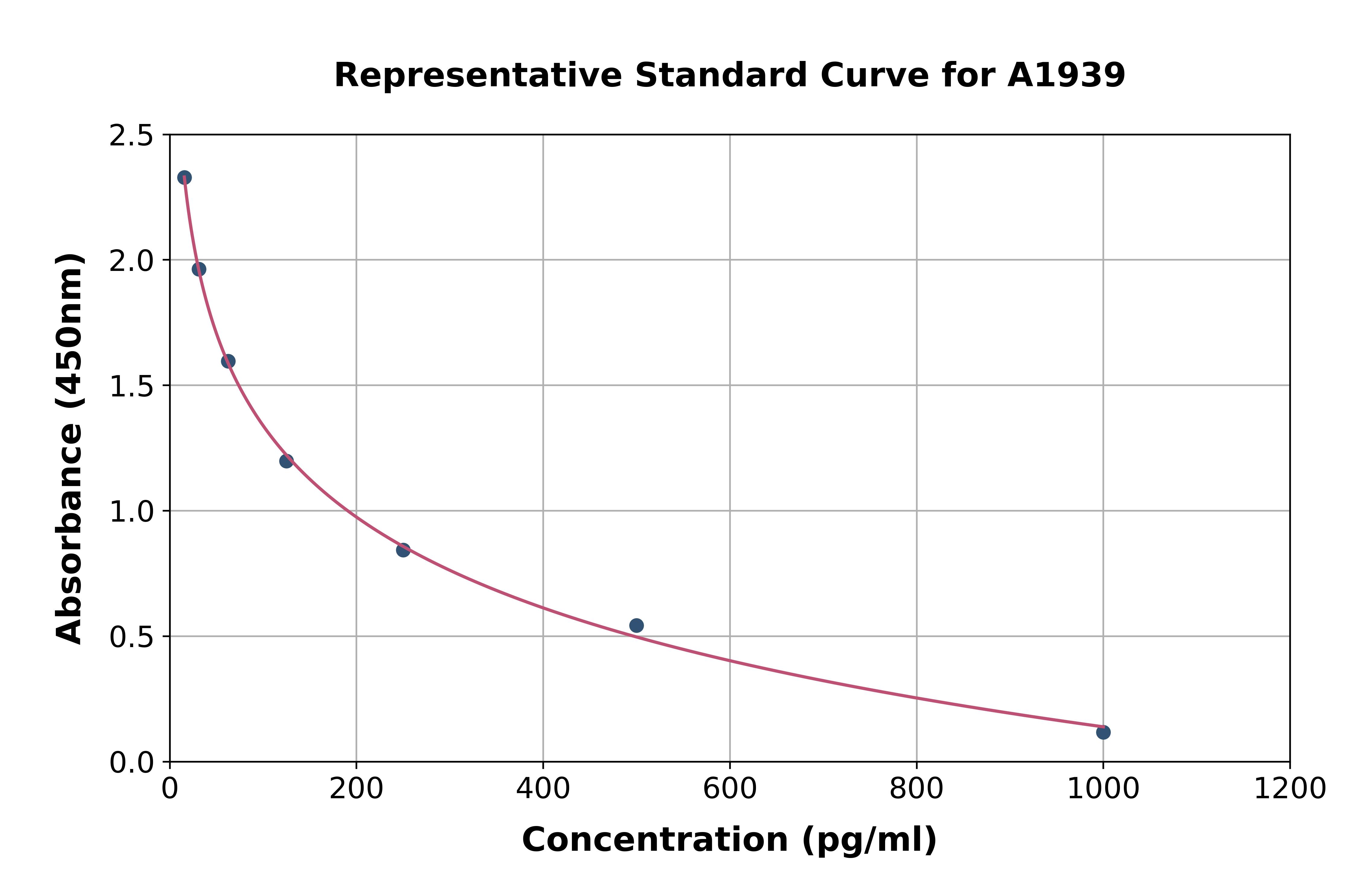
Angiotensin I ELISA kit
A05882
Product group Assays
Overview
- SupplierBertin Bioreagent
- Product NameAngiotensin I ELISA kit
- Delivery Days Customer4
- ApplicationsELISA
- Assay Detection Range16-2000 pg/mL
- CertificationResearch Use Only
- ConjugateAChE
- Scientific DescriptionAngiotensin I ELISA kit
- Shelf life instructionStore at -20degrees;shelf life 2 years maximum after production
- Storage Instruction-20°C
- UNSPSC41116133