ANGPTL4 (fibrinogen-like domain) (human) (rec.)
AG-40A-0070
Protein IDQ9BY76
Product group Proteins / Signaling Molecules
Overview
- SupplierAdipoGen Life Sciences
- Product NameANGPTL4 (fibrinogen-like domain) (human) (rec.)
- Delivery Days Customer10
- CertificationResearch Use Only
- Concentration0.5 mg/ml
- Estimated Purity>90%
- Gene ID51129
- Target nameANGPTL4
- Target descriptionangiopoietin like 4
- Target synonymsARP4, FIAF, HARP, HFARP, NL2, PGAR, TGQTL, UNQ171, pp1158, angiopoietin-related protein 4, PPARG angiopoietin related protein, fasting-induced adipose factor, hepatic angiopoietin-related protein, hepatic fibrinogen/angiopoietin-related protein, peroxisome proliferator-activated receptor (PPAR) gamma induced angiopoietin-related protein
- Protein IDQ9BY76
- Protein NameAngiopoietin-related protein 4
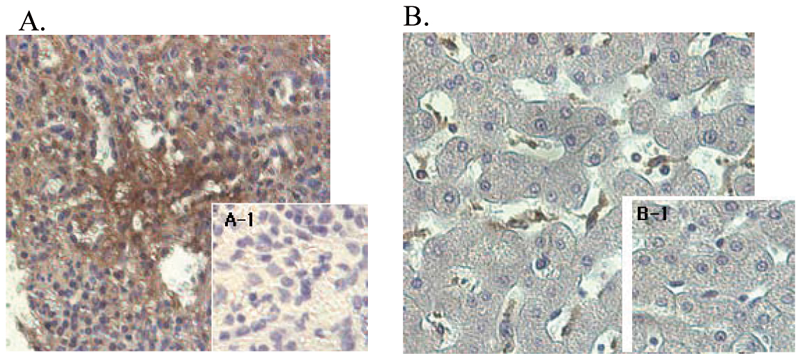
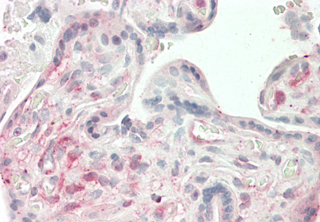
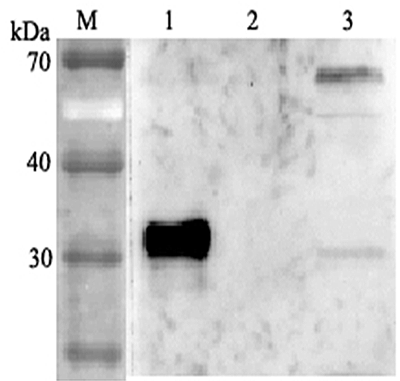
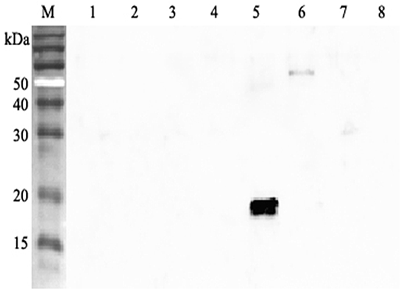
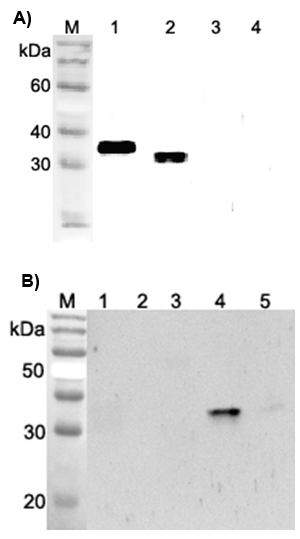
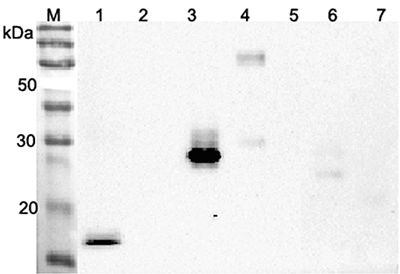
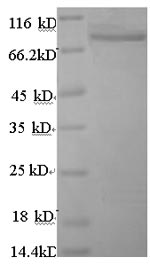
- Scientific DescriptionANGPTL4 (Angiopoietin-like protein 4) mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorigenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7. - Protein. Fibrinogen-like domain of human ANGPTL4 (aa 166-406) is fused at the N-terminus to a FLAG®-tag. Source: HEK 293 cells. Endotoxin content: 90% (SDS-PAGE). ANGPTL4 (Angiopoietin-like protein 4) mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorigenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7.
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116100
- SpeciesHuman