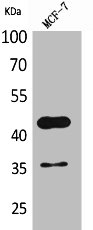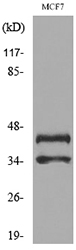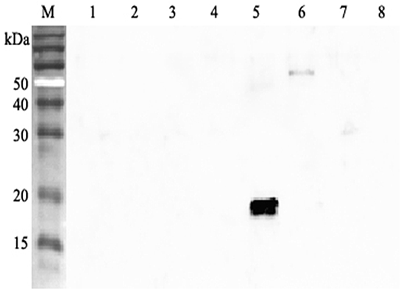
Western blot analysis using anti-ANGPTL4 (human), pAb (Prod. No. AG-25A-0055) at 1:2000 dilution. 1: Human ANGPTL1 (FLAG®-tagged). 2: Human ANGPTL2 (FLAG®-tagged). 3: Human ANGPTL3 (FLAG®-tagged).
anti-ANGPTL4 (human), pAb
AG-25A-0055
ApplicationsWestern Blot, ELISA
Product group Antibodies
ReactivityHuman
TargetANGPTL4
Overview
- SupplierAdipoGen Life Sciences
- Product Nameanti-ANGPTL4 (human), pAb
- Delivery Days Customer10
- ApplicationsWestern Blot, ELISA
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration1 mg/ml
- Gene ID51129
- Target nameANGPTL4
- Target descriptionangiopoietin like 4
- Target synonymsARP4, FIAF, HARP, HFARP, NL2, PGAR, TGQTL, UNQ171, pp1158, angiopoietin-related protein 4, PPARG angiopoietin related protein, fasting-induced adipose factor, hepatic angiopoietin-related protein, hepatic fibrinogen/angiopoietin-related protein, peroxisome proliferator-activated receptor (PPAR) gamma induced angiopoietin-related protein
- HostRabbit
- Protein IDQ9BY76
- Protein NameAngiopoietin-related protein 4
- Scientific DescriptionANGPTL4 mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorgenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7. - Polyclonal Antibody. Recognizes the coiled-coil domain of human ANGPTL4. Detects a band of ~18kDa by Western blot. Weakly cross-reacts with human ANGPTL6. Does not cross-react with other ANGPTL family proteins. Source: Rabbit. Applications: ELISA, WB. Liquid. 0.2microm-filtered solution in PBS, pH 7.4. Contains no preservatives. ANGPTL4 mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorgenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7.
- ReactivityHuman
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161