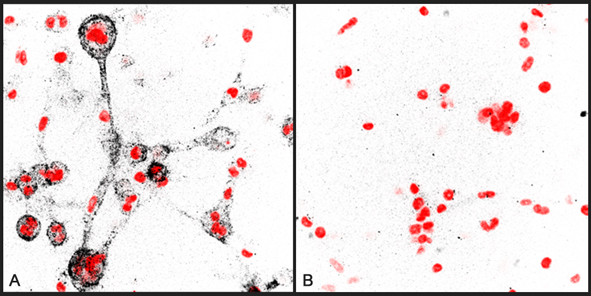
Immunocytochemical staining of collagen degradation by human MDA-MB-231 breast cancer cells embedded in a 3D typeI collagen matrix using anti-Collagen Type 1 (3/4 fragment), pAb (Prod. No. AG-25T-0116). Cells have been treated with non-targeting siRNA(A) o
anti-Collagen Type 1 (3/4 fragment), pAb
AG-25T-0116
ApplicationsWestern Blot, ImmunoCytoChemistry
Product group Antibodies
ReactivityBovine, Canine, Feline, Hamster, Human, Mouse, Porcine, Rat, Sheep
Overview
- SupplierAdipoGen Life Sciences
- Product Nameanti-Collagen Type 1 (3/4 fragment), pAb
- Delivery Days Customer7
- ApplicationsWestern Blot, ImmunoCytoChemistry
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration250 ug/ml
- HostRabbit
- Scientific DescriptionPolyclonal Antibody. Recognizes rat and bovine collagen type 1. Based on sequence identity should also recognize human, mouse, guinea pig, dog, cat, donkey, pig, cow, sheep and chicken collagen type 1. Source: Rabbit. Applications: ICC. Liquid. In PBS with 1 mg/ml BSA and 0.02% sodium azide. The proteolysis of collagens plays an important role in numerous physiological and pathological situations such as morphogenesis, wound healing, arthritis, arteriosclerosis and tumor metastasis. Triple helical type I collagens are made up of two alpha1 (I) and one alpha2 (I) chains, and are found in skin, tendon, ligament and interstitial tissues. Due to their fibrillary structure native collagens are resistant to most proteases. They are substrates for certain matrix metalloproteinases (MMPs), which constitute a family of zinc-dependent enzymes catalyzing the degradation of extracellular matrix components. Initial MMP-8 dependent cleavage of collagen into the characteristic 3/4 and 1/4 fragments has been shown to enable MMP-9 diffusion along the protein helix with preferential binding to the collagen 3/4 fragment tail. Finally, untwisting of the helix end results in the local denaturation of the triple helical structure. - The proteolysis of collagens plays an important role in numerous physiological and pathological situations such as morphogenesis, wound healing, arthritis, arteriosclerosis and tumor metastasis. Triple helical type I collagens are made up of two alpha1 (I) and one alpha2 (I) chains, and are found in skin, tendon, ligament and interstitial tissues. Due to their fibrillary structure native collagens are resistant to most proteases. They are substrates for certain matrix metalloproteinases (MMPs), which constitute a family of zinc-dependent enzymes catalyzing the degradation of extracellular matrix components. Initial MMP-8 dependent cleavage of collagen into the characteristic 3/4 and 1/4 fragments has been shown to enable MMP-9 diffusion along the protein helix with preferential binding to the collagen 3/4 fragment tail. Finally, untwisting of the helix end results in the local denaturation of the triple helical structure.
- ReactivityBovine, Canine, Feline, Hamster, Human, Mouse, Porcine, Rat, Sheep
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161
