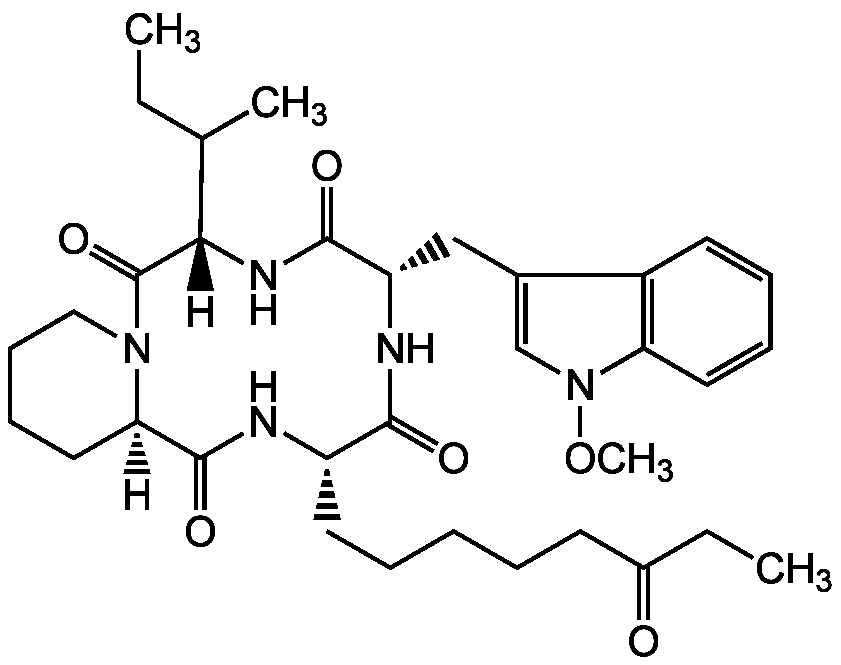
Chemical Structure
Apicidin [183506-66-3]
AG-CN2-0087
CAS Number183506-66-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight623.8
Overview
- SupplierAdipoGen Life Sciences
- Product NameApicidin [183506-66-3]
- Delivery Days Customer10
- CAS Number183506-66-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC34H49N5O6
- Molecular Weight623.8
- Scientific DescriptionAntiprotozoal [1]. Potent inhibitor of histone deacetylase (HDAC) [1,2]. Inhibits proliferation [2,3]. Induces cell cycle arrest at the G1 phase [2]. Induces hyperacetylation of histone H4 [5]. Apoptosis inducer [4,8,9]. Potent anti-angiogenic compound [5-7]. Decreases HIF-1alpha protein levels and transcriptional activity in human and mouse tumor cell lines [3]. Autophagy inducer in oral squamous cell carcinoma (OSCC) cells [8]. Promotes either self-renewal or differentiation of embryonic stem cells [10]. - Chemical. CAS: 183506-66-3. Formula: C34H49N5O6. MW: 623.8. Isolated from fungus Fusarium sp. Antiprotozoal. Potent inhibitor of histone deacetylase (HDAC). Inhibits proliferation. Induces cell cycle arrest at the G1 phase. Induces hyperacetylation of histone H4. Apoptosis inducer. Potent anti-angiogenic compound. Decreases HIF-1alpha protein levels and transcriptional activity in human and mouse tumor cell lines. Autophagy inducer in oral squamous cell carcinoma (OSCC) cells. Promotes either self-renewal or differentiation of embryonic stem cells.
- SMILES[H][C@]12CCCCN1C(=O)[C@@]([H])(NC(=O)[C@H](CC1=CN(OC)C3=C1C=CC=C3)NC(=O)[C@H](CCCCCC(=O)CC)NC2=O)C(C)CC
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200