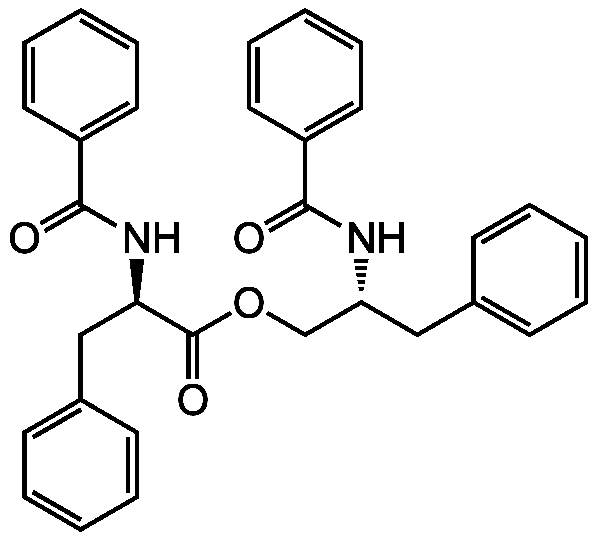
Chemical Structure
Asperphenamate [63631-36-7]
AG-CN2-0171
CAS Number63631-36-7
Product group Chemicals
Estimated Purity>95%
Molecular Weight506.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameAsperphenamate [63631-36-7]
- Delivery Days Customer10
- CAS Number63631-36-7
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC32H30N2O4
- Molecular Weight506.6
- Scientific DescriptionAnticancer compound. Cytotoxic against human breast cancer cells. Induces autophagic cell death in MCF-7 cells. Moderate radical scavenger. Weak acetylcholinesterase (AChE) inhibitor. Shows moderate trypanocidal activity. - Chemical. CAS: 63631-36-7. Formula: C32H30N2O4. MW: 506.6. Isolated from Aspergillus sp. Anticancer compound. Cytotoxic against human breast cancer cells. Induces autophagic cell death in MCF-7 cells. Moderate radical scavenger. Weak acetylcholinesterase (AChE) inhibitor. Shows moderate trypanocidal activity.
- SMILESO=C(OC[C@@H](CC1=CC=CC=C1)NC(=O)C1=CC=CC=C1)[C@@H](CC1=CC=CC=C1)NC(=O)C1=CC=CC=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Asperphenamate [63631-36-7]](https://www.targetmol.com/group3/M00/36/C0/CgoaEGayQySETRWDAAAAALmD0KE486.png)