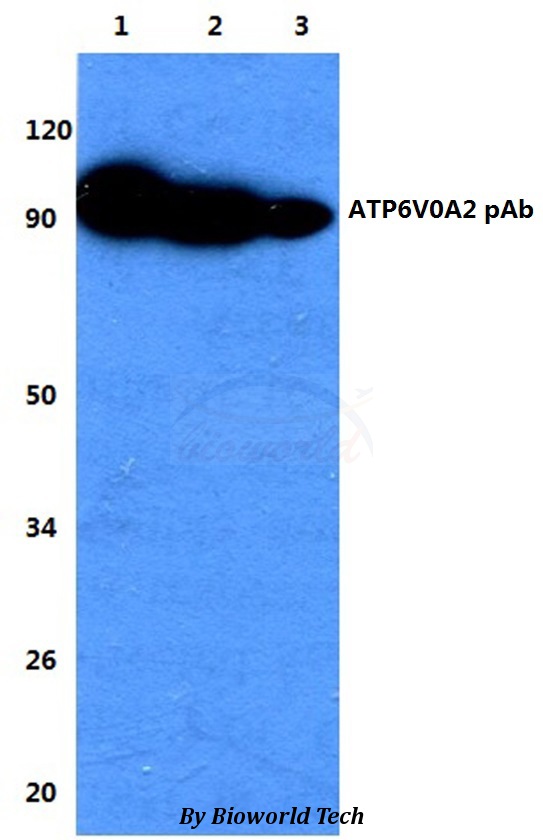
ATP6V0A2 antibody [N1N3]
GTX111275
ApplicationsWestern Blot
Product group Antibodies
ReactivityHuman
TargetATP6V0A2
Overview
- SupplierGeneTex
- Product NameATP6V0A2 antibody [N1N3]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:500-1:3000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsWestern Blot
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration0.89 mg/ml
- ConjugateUnconjugated
- Gene ID23545
- Target nameATP6V0A2
- Target descriptionATPase H+ transporting V0 subunit a2
- Target synonymsA2, ARCL, ARCL2A, ATP6A2, ATP6N1D, J6B7, RTF, STV1, TJ6, TJ6M, TJ6S, VPH1, WSS, a2V, V-type proton ATPase 116 kDa subunit a 2, A2V-ATPase, ATPase, H+ transporting, lysosomal V0 subunit a2, V-ATPase 116 kDa subunit a 2, V-ATPase 116 kDa subunit a2, V-ATPase subunit a2, V-type proton ATPase 116 kDa subunit a2, lysosomal H(+)-transporting ATPase V0 subunit a 2, lysosomal H(+)-transporting ATPase V0 subunit a2, regeneration and tolerance factor, v-type proton ATPase 116 kDa subunit a, vacuolar proton translocating ATPase 116 kDa subunit a
- HostRabbit
- IsotypeIgG
- Protein IDQ9Y487
- Protein NameV-type proton ATPase 116 kDa subunit a 2
- Scientific DescriptionThe multisubunit vacuolar-type proton pump (H(+)-ATPase or V-ATPase) is essential for acidification of diverse cellular components, including endosomes, lysosomes, clathrin-coated vesicles, secretory vesicles, and chromaffin granules, and it is found at high density in the plasma membrane of certain specialized cells. H(+)-ATPases are comprised of a peripheral V(1) domain and an integral membrane V(0) domain; ATP6V0A2 is a component of the V(0) domain (Smith et al., 2003 [PubMed 14580332]).[supplied by OMIM]
- ReactivityHuman
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203
References
- Zhang X, Cao J, Miller SP, et al. Comparative Nucleotide-Dependent Interactome Analysis Reveals Shared and Differential Properties of KRas4a and KRas4b. ACS Cent Sci. 2018,4(1):71-80. doi: 10.1021/acscentsci.7b00440Read this paper
- Matsumoto A, Pasut A, Matsumoto M, et al. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017,541(7636):228-232. doi: 10.1038/nature21034Read this paper