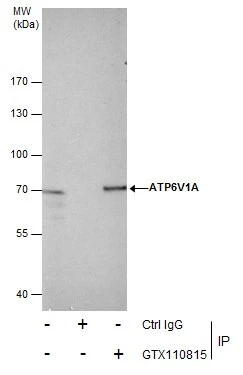
Immunoprecipitation of ATP6V1A protein from HeLa whole cell extracts using 5 μg of ATP6V1A antibody (GTX110815). Western blot analysis was performed using ATP6V1A antibody (GTX110815). EasyBlot anti-Rabbit IgG (GTX221666-01) was used as a secondary reagent.
ATP6V1A antibody
GTX110815
ApplicationsImmunoPrecipitation, Western Blot, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
Product group Antibodies
ReactivityHuman, Mouse
TargetATP6V1A
Overview
- SupplierGeneTex
- Product NameATP6V1A antibody
- Delivery Days Customer9
- Application Supplier NoteWB: 1:1000-1:10000. IHC-P: 1:100-1:1000. IP: 1:100-1:500. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoPrecipitation, Western Blot, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration0.57 mg/ml
- ConjugateUnconjugated
- Gene ID523
- Target nameATP6V1A
- Target descriptionATPase H+ transporting V1 subunit A
- Target synonymsARCL2D, ATP6A1, ATP6V1A1, DEE93, HO68, IECEE3, VA68, VPP2, Vma1, V-type proton ATPase catalytic subunit A, ATPase, H+ transporting, lysosomal 70kDa, V1 subunit A, ATPase, H+ transporting, lysosomal, subunit A1, H(+)-transporting two-sector ATPase, subunit A, H+-transporting ATPase chain A, vacuolar (VA68 type), V-ATPase 69 kDa subunit 1, V-ATPase A subunit 1, V-ATPase subunit A, V-type proton ATPase (V-ATPase) catalytic subunit A, vacuolar proton pump alpha subunit 1, vacuolar proton pump subunit alpha, vacuolar-type H(+)-ATPase
- HostRabbit
- IsotypeIgG
- Protein IDP38606
- Protein NameV-type proton ATPase catalytic subunit A
- Scientific DescriptionThis gene encodes a component of vacuolar ATPase (V-ATPase), a multisubunit enzyme that mediates acidification of eukaryotic intracellular organelles. V-ATPase dependent organelle acidification is necessary for such intracellular processes as protein sorting, zymogen activation, receptor-mediated endocytosis, and synaptic vesicle proton gradient generation. V-ATPase is composed of a cytosolic V1 domain and a transmembrane V0 domain. The V1 domain consists of three A and three B subunits, two G subunits plus the C, D, E, F, and H subunits. The V1 domain contains the ATP catalytic site. The V0 domain consists of five different subunits: a, c, c, c, and d. Additional isoforms of many of the V1 and V0 subunit proteins are encoded by multiple genes or alternatively spliced transcript variants. This encoded protein is one of two V1 domain A subunit isoforms and is found in all tissues. Transcript variants derived from alternative polyadenylation exist. [provided by RefSeq]
- ReactivityHuman, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161

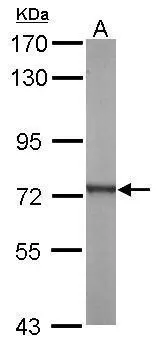
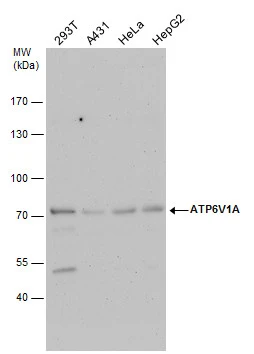
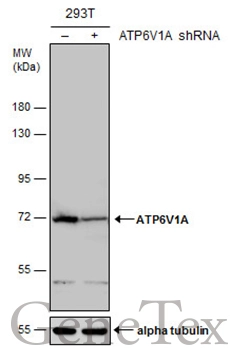



![Non-transfected (–) and transfected (+) 293T whole cell extracts (30 μg) were separated by 7.5% SDS-PAGE, and the membrane was blotted with ATP6V1A antibody [GT3846] (GTX633542) diluted at 1:500.](https://www.genetex.com/upload/website/prouct_img/normal/GTX633542/GTX633542_42520_20161013_WB_shRNA_watermark_w_23061202_284.webp)
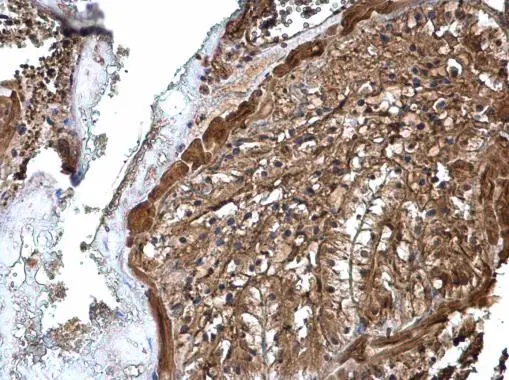
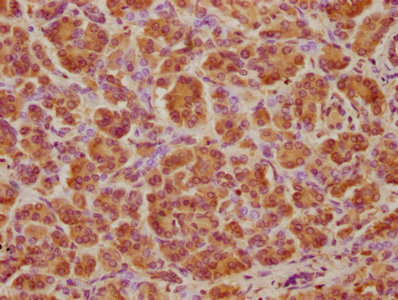
![ATP6V1A antibody [GT811] detects ATP6V1A protein at cytoplasm by immunohistochemical analysis. Sample: Paraffin-embedded rat liver. ATP6V1A stained by ATP6V1A antibody [GT811] (GTX633543) diluted at 1:200. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min](https://www.genetex.com/upload/website/prouct_img/normal/GTX633543/GTX633543_44699_20220603_IHC-P_R_22062919_641.webp)
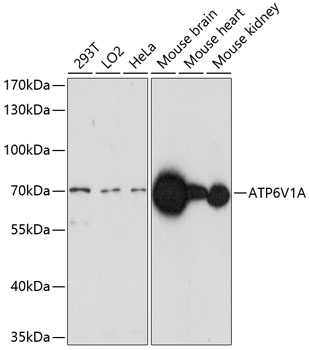
![Non-transfected (–) and transfected (+) 293T whole cell extracts (30 μg) were separated by 7.5% SDS-PAGE, and the membrane was blotted with ATP6V1A antibody [GT1561] (GTX633544) diluted at 1:500.](https://www.genetex.com/upload/website/prouct_img/normal/GTX633544/GTX633544_42569_20161013_WB_shRNA_watermark_w_23061202_116.webp)