Chemical Structure
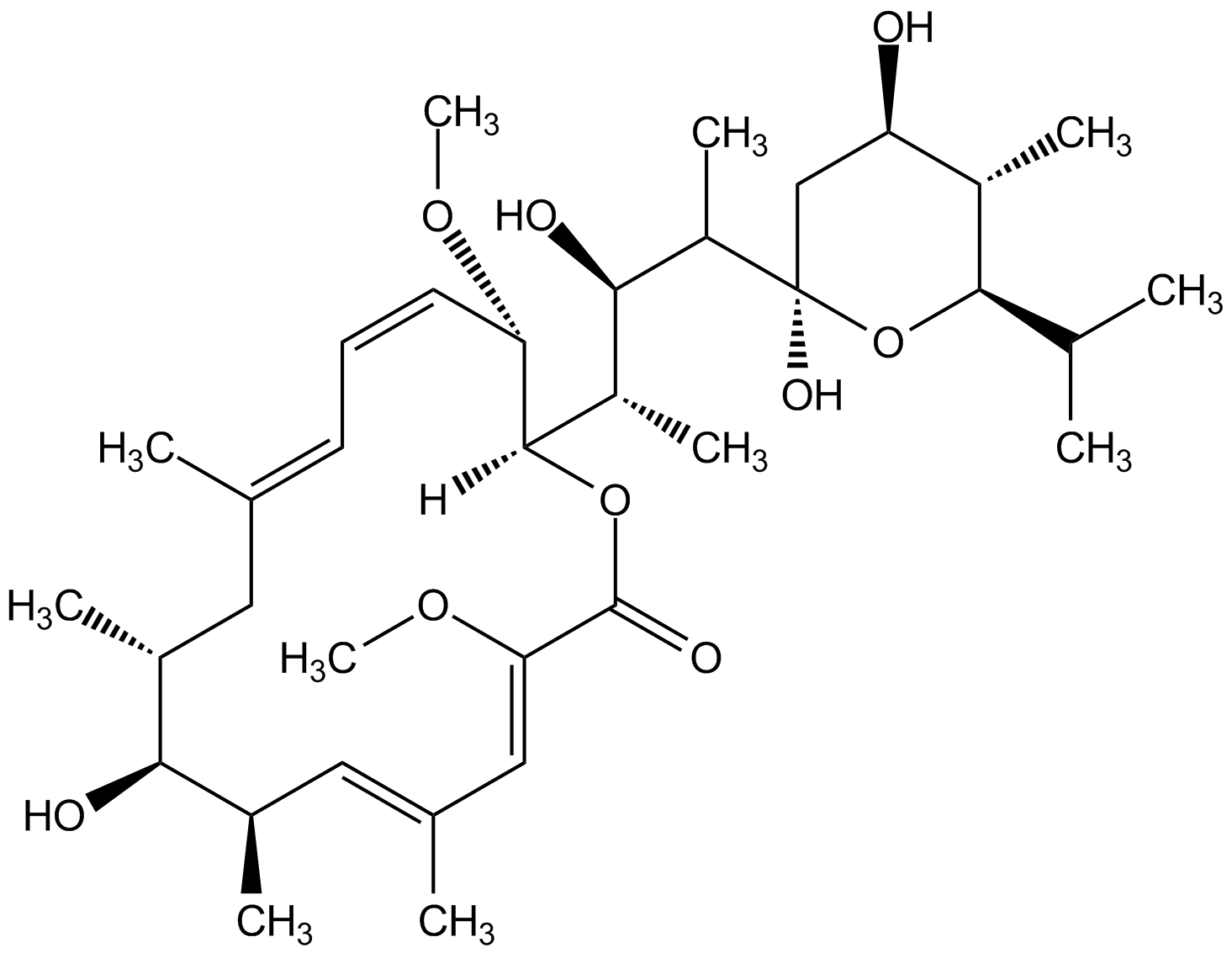
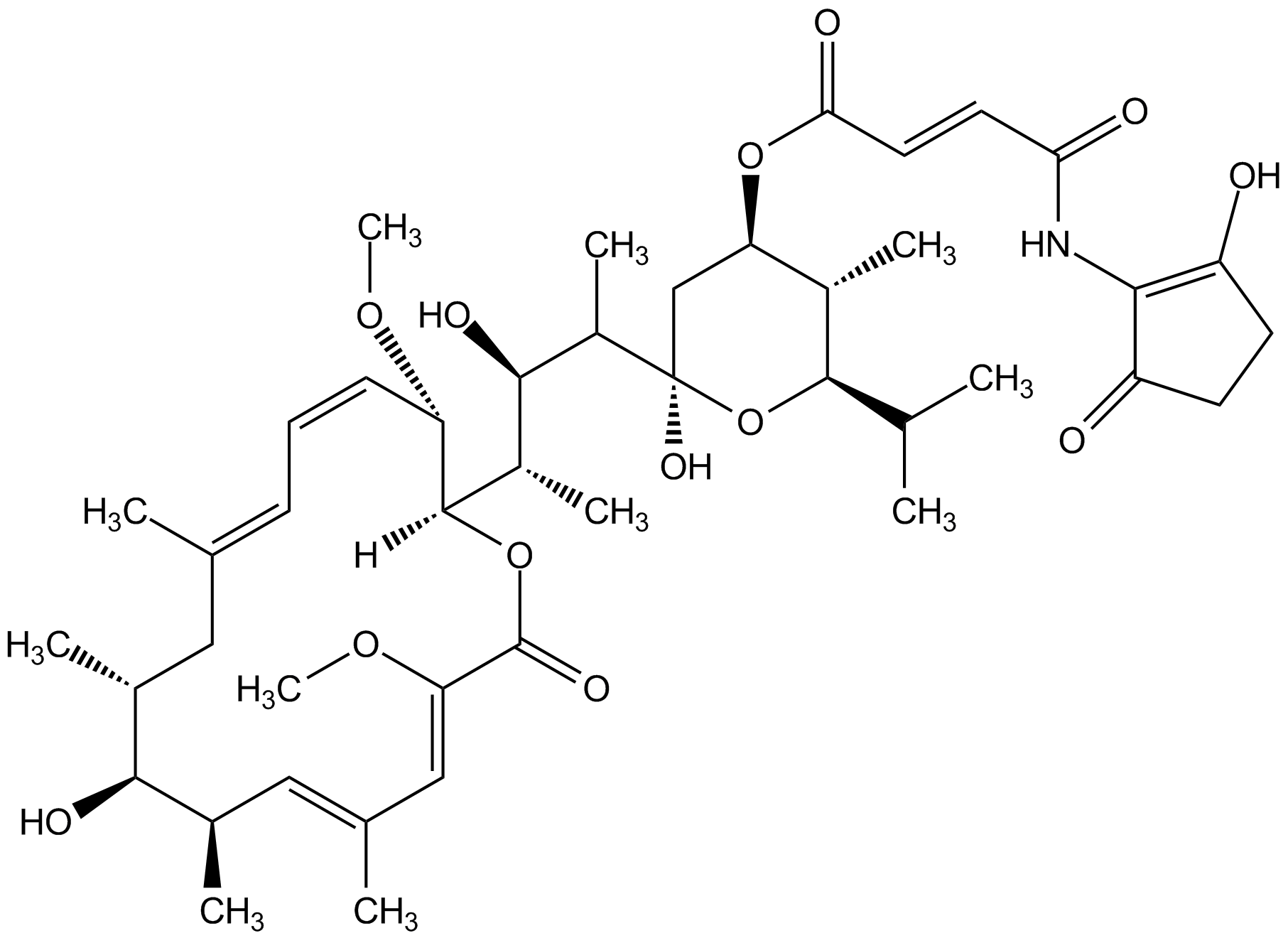
Bafilomycin B1 [88899-56-3] [88899-56-3]
BVT-0004
CAS Number88899-56-3
Product group Chemicals
Estimated Purity>97%
Molecular Weight815.9
Overview
- SupplierBioViotica
- Product NameBafilomycin B1 [88899-56-3] [88899-56-3]
- Delivery Days Customer2
- CAS Number88899-56-3
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC44H65NO13
- Molecular Weight815.9
- Scientific DescriptionChemical. CAS: 88899-56-3. Formula: C44H65NO13. MW: 815.9. Isolated from Streptomyces hygroscopicus. Macrolide antibiotic. Specific vacuolar-type H+-ATPase inhibitor. Inhibitor of autophagic degradation by rising lysosomal pH and thus inactivating the lysosomal acid hydrolases. Antitrypanosomal and antileishmanial compound. Phytotoxic. Influences the cell wall biosynthesis of Asp. niger. - Macrolide antibiotic. Specific vacuolar-type H+-ATPase inhibitor. Inhibitor of autophagic degradation by rising lysosomal pH and thus inactivating the lysosomal acid hydrolases. Antitrypanosomal and antileishmanial compound. Phytotoxic. Influences the cell wall biosynthesis of Asp. niger.
- SMILES[H][C@@]1(OC(=O)\C(OC)=C\C(\C)=C\[C@@H](C)[C@@H](O)[C@@H](C)C\C(C)=C\C=C/[C@@H]1OC)[C@@H](C)[C@@H](O)[C@H](C)[C@@]1(O)C[C@@H](OC(=O)\C=C\C(=O)NC2=C(O)CCC2=O)[C@H](C)C(O1)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200

![Bafilomycin B1 [88899-56-3]](https://www.targetmol.com/group3/M00/36/BE/CgoaEGayQwOETVGnAAAAABix4KI321.png)