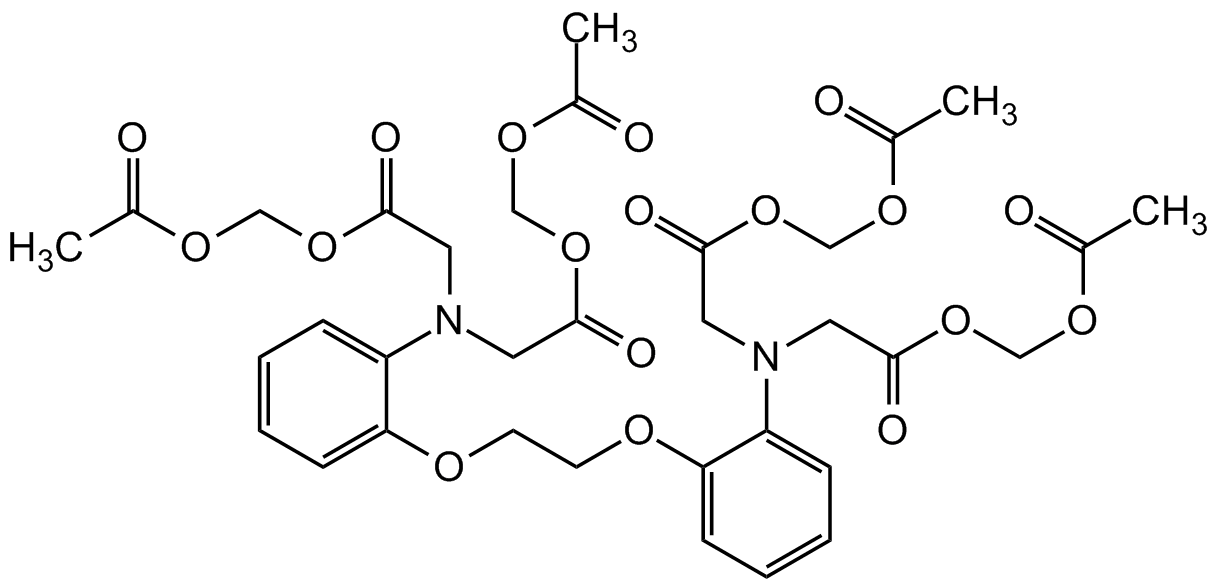
Chemical Structure
BAPTA-AM [126150-97-8] [126150-97-8]
CDX-B0285
CAS Number126150-97-8
Product group Chemicals
Estimated Purity>95%
Molecular Weight764.68
Overview
- SupplierChemodex
- Product NameBAPTA-AM [126150-97-8] [126150-97-8]
- Delivery Days Customer2
- CAS Number126150-97-8
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC34H40N2O18
- Molecular Weight764.68
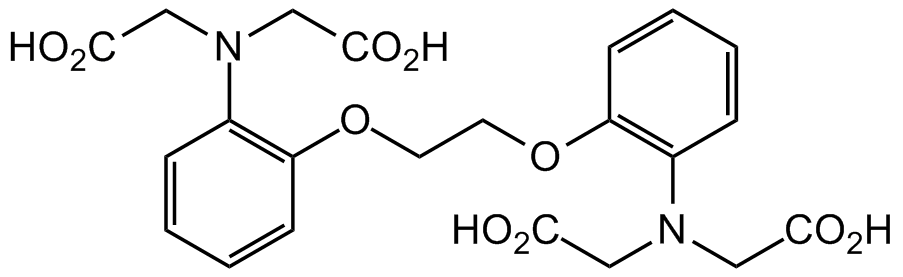
- Scientific DescriptionBAPTA is a membrane-impermeable highly selective calcium chelator and is useful for manipulating extracellular Ca2+ levels, with 105-fold greater affinity for Ca2+ than for Mg2+. The presence of four carboxylic acid functional groups allows for the binding of two calcium ions. BAPTA and its derivatives can be used as calcium indicators, since the absorption maximum for BAPTA changes when it is complexed with calcium (absorption maxima free/complexed = 254/274 nm, emission maxima free/complexed = 363/363 nm). Key advantages of BAPTA include relative insensitivity toward intracellular pH change and fast release of calcium. This product is a high quality and sensitive compound for calcium signaling studies, investigation of signal transduction, apoptotic cascades and neuroscience research. BAPTA AM is a membrane permeable analog of BAPTA that binds calcium only after the acetoxymethyl group is removed by cytoplasmic esterases. BAPTA AM ester itself does not bind calcium, but once inside the cell is converted into BAPTA by cytoplasmic esterases. BAPTA-AM is useful for controlling the intracellular calcium concentration and it is commonly used at 10-100 microM for this purpose. It can also be used in animals. BAPTA AM also inhibits voltage-gated potassium (Kv) channels, including Kv1.3, Kv1.5 and Kv11.1 (Ki = 1.45, 1.23, and 1.30 microM, respectively). BAPTA-AM inhibits free radical-mediated toxicity, enhances apoptosis in non-neuronal cells and protects neurons from ischemic damage. It regulates ion channels and blocks neuronal Ca2+-activated K+ channel currents. BAPTA-AM maintains intracellular Ca2+ homeostasis. - Chemical. CAS: 126150-97-8. Formula: C34H40N2O18. MW: 764.68. BAPTA is a membrane-impermeable highly selective calcium chelator and is useful for manipulating extracellular Ca2+ levels, with 105-fold greater affinity for Ca2+ than for Mg2+. The presence of four carboxylic acid functional groups allows for the binding of two calcium ions. BAPTA and its derivatives can be used as calcium indicators, since the absorption maximum for BAPTA changes when it is complexed with calcium (absorption maxima free/complexed = 254/274 nm, emission maxima free/complexed = 363/363 nm). Key advantages of BAPTA include relative insensitivity toward intracellular pH change and fast release of calcium. This product is a high quality and sensitive compound for calcium signaling studies, investigation of signal transduction, apoptotic cascades and neuroscience research. BAPTA AM is a membrane permeable analog of BAPTA that binds calcium only after the acetoxymethyl group is removed by cytoplasmic esterases. BAPTA AM ester itself does not bind calcium, but once inside the cell is converted into BAPTA by cytoplasmic esterases. BAPTA-AM is useful for controlling the intracellular calcium concentration and it is commonly used at 10-100 microM for this purpose. It can also be used in animals. BAPTA AM also inhibits voltage-gated potassium (Kv) channels, including Kv1.3, Kv1.5 and Kv11.1 (Ki = 1.45, 1.23, and 1.30 microM, respectively). BAPTA-AM inhibits free radical-mediated toxicity, enhances apoptosis in non-neuronal cells and protects neurons from ischemic damage. It regulates ion channels and blocks neuronal Ca2+-activated K+ channel currents. BAPTA-AM maintains intracellular Ca2+ homeostasis.
- SMILESO=C(OCOC(C)=O)CN(CC(OCOC(C)=O)=O)C1=CC=CC=C1OCCOC2=CC=CC=C2N(CC(OCOC(C)=O)=O)CC(OCOC(C)=O)=O
- Storage Instruction-20°C
- UNSPSC12162000


![BAPTA-AM [126150-97-8] [126150-97-8]](https://www.targetmol.com/group3/M00/35/80/CgoaEGayIYSEQox-AAAAAJw-5aE874.png)