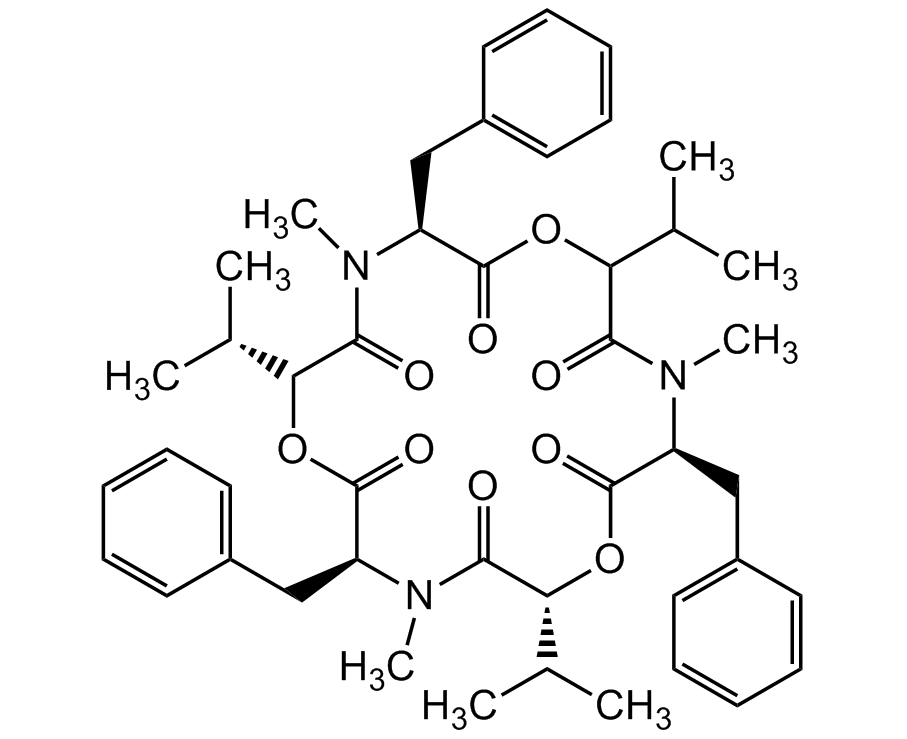
Chemical Structure
Beauvericin [26048-05-5]
AG-CN2-0043
CAS Number26048-05-5
Product group Chemicals
Estimated Purity>97%
Molecular Weight784
Overview
- SupplierAdipoGen Life Sciences
- Product NameBeauvericin [26048-05-5]
- Delivery Days Customer10
- CAS Number26048-05-5
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC45H57N3O9
- Molecular Weight784
- Scientific DescriptionAntibiotic [4]. Mycotoxin. Apoptosis inducer [1, 3, 8, 9, 18]. Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor [2]. Anticancer compound [3, 7, 9]. Antihaptotactic and antimetastatic [10]. Antiangiogenic compound [10]. Antibacterial, antiprotozal, antiviral and antifungal compound [4, 11, 13, 15, 16]. Shows ionophoric properties [5, 17]. Cytotoxic [6, 7]. Genotoxic [14]. Potently interacts with ABCB1 and ABCG2 transport functions [12]. Causes mitochondrial dysfunction [17]. - Chemical. CAS: 26048-05-5. Formula: C45H57N3O9. MW: 784. Isolated from fungus Beauveria sp. Antibiotic. Apoptosis inducer. Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor. Anticancer compound. Antihaptotactic and antimetastatic. Antiangiogenic compound. Antibacterial, antiprotozal, antiviral and antifungal compound. Shows ionophoric properties. Cytotoxic. Genotoxic. Potently interacts with ABCB1 and ABCG2 transport functions. Causes mitochondrial dysfunction.
- SMILESCC.CC(C)C1OC(=O)[C@H](CC2=CC=CC=C2)N(C)C(=O)C(OC(=O)[C@H](CC2=CC=CC=C2)N(C)C(=O)[C@H](OC(=O)[C@H](CC2=CC=CC=C2)N(C)C1=O)C(C)C)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Beauvericin [26048-05-5]](https://www.targetmol.com/group3/M00/36/C4/CgoaEWayQ4uEDyyOAAAAAHjxAgc333.png)