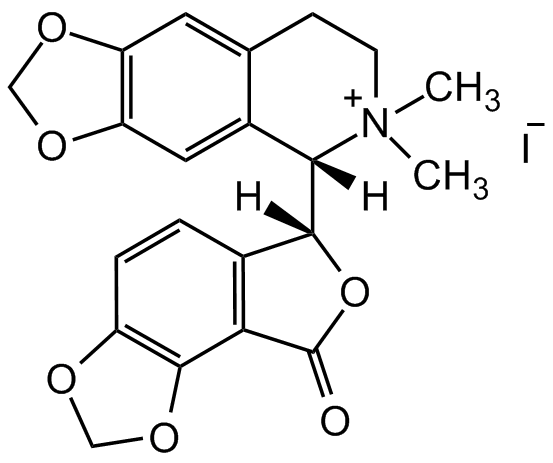
Chemical Structure
(-)-Bicuculline methiodide [40709-69-1] [40709-69-1]
CDX-B0240
CAS Number40709-69-1
Product group Chemicals
Estimated Purity>95%
Molecular Weight509.29
Overview
- SupplierChemodex
- Product Name(-)-Bicuculline methiodide [40709-69-1] [40709-69-1]
- Delivery Days Customer2
- CAS Number40709-69-1
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC21H20INO6
- Molecular Weight509.29
- Scientific Description(-)-Bicuculline methiodide is the N-methylated water-soluble derivative of the widely-employed ionotropic GABAA receptor antagonist (+)-Bicuculline. This prototypic, competitive GABAA receptor antagonist displaces GABA from the agonist binding site to prevent receptor activation. It also acts as a negative allosteric inhibitor of channel opening to inhibit GABAA receptor activation by anaesthetic agents. Additionally it shows activity at SK calcium-activated potassium channels, nicotinic acetylcholine receptors and acetylcholinesterase. This compound reversibly and competitively blocks GABAA receptor mediated currents and is widely used to isolate glutamate receptor mediated EPSCs (excitatory postsynaptic potentials). - Chemical. CAS: 40709-69-1. Formula: C21H20INO6. MW: 509.29. (-)-Bicuculline methiodide is the N-methylated water-soluble derivative of the widely-employed ionotropic GABAA receptor antagonist (+)-Bicuculline. This prototypic, competitive GABAA receptor antagonist displaces GABA from the agonist binding site to prevent receptor activation. It also acts as a negative allosteric inhibitor of channel opening to inhibit GABAA receptor activation by anaesthetic agents. Additionally it shows activity at SK calcium-activated potassium channels, nicotinic acetylcholine receptors and acetylcholinesterase. This compound reversibly and competitively blocks GABAA receptor mediated currents and is widely used to isolate glutamate receptor mediated EPSCs (excitatory postsynaptic potentials).
- SMILESC[N+]1(C)[C@@]([C@@]2([H])C(C=CC3=C4OCO3)=C4C(O2)=O)([H])C5=CC6=C(OCO6)C=C5CC1.[I-]
- Storage Instruction2°C to 8°C,RT
- UN Number1544
- UNSPSC12352200

![(+)-Bicuculline methiodide [40709-69-1]](https://www.targetmol.com/group3/M00/02/43/CgoaEGY7LiaEbc9vAAAAAF3XkPc359.png)