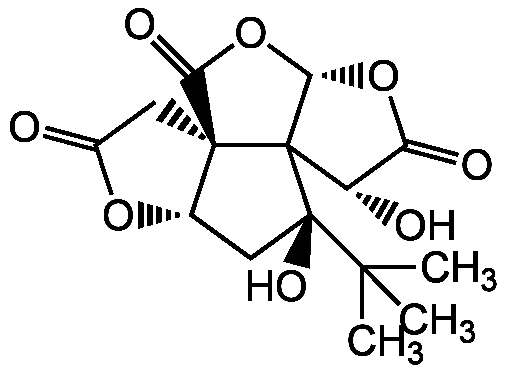
Chemical Structure
Bilobalide [33570-04-6] [33570-04-6]
AG-CN2-0026
CAS Number33570-04-6
Product group Chemicals
Estimated Purity>95%
Molecular Weight326.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameBilobalide [33570-04-6] [33570-04-6]
- Delivery Days Customer10
- CAS Number33570-04-6
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC15H18O8
- Molecular Weight326.3
- Scientific DescriptionChemical. CAS: 33570-04-6. Formula: C15H18O8. MW: 326.3. Isolated from Ginko biloba. Neuroprotective. Mitochondrial gene expression regulator. ROS scavenger. Competitive GABA(A) receptor antagonist. Apoptosis inhibitor. CREB phosphorylation enhancer. Stimulates neurogenesis and synaptogenesis. Hepatic cytochrome P450 inducer. Activates the phosphatidylinositol 3-kinase (PI3K) dependent pathway. Potent anti-inflammatory and antihyperalgesic agent. - Neuroprotective [2, 3, 4, 5, 7]. Mitochondrial gene expression regulator [3]. ROS scavenger [4]. Competitive GABA(A) receptor antagonist [6]. Apoptosis inhibitor [4, 7, 10, 11]. CREB phosphorylation enhancer. Stimulates neurogenesis and synaptogenesis [8]. Hepatic cytochrome P450 inducer [9]. Activates the phosphatidylinositol 3-kinase (PI3K) dependent pathway [10, 11]. Potent anti-inflammatory and antihyperalgesic agent [12].
- SMILESCC(C)(C)[C@]1(O)C[C@@H]2OC(=O)C[C@@]22C(=O)O[C@@H]3OC(=O)[C@H](O)C123
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Bilobalide [33570-04-6] [33570-04-6]](https://www.targetmol.com/group3/M00/35/43/CgoaEGayGjmEW9RDAAAAALtBLME202.png)