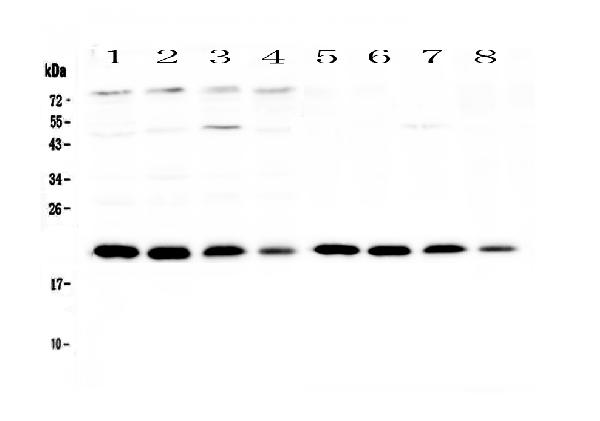
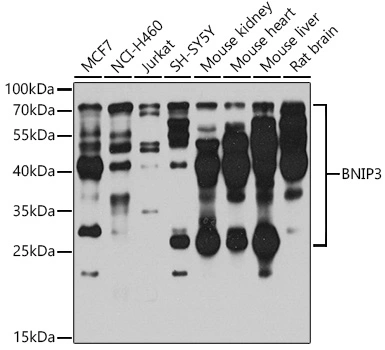
BNIP3 antibody [ANa40]
GTX10433
ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ELISA, ImmunoCytoChemistry, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman
TargetBNIP3
Overview
- SupplierGeneTex
- Product NameBNIP3 antibody [ANa40]
- Delivery Days Customer9
- Application Supplier NoteWB: 2-4 microg/ml. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ELISA, ImmunoCytoChemistry, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDANa40
- Concentration2 mg/ml
- ConjugateUnconjugated
- Gene ID664
- Target nameBNIP3
- Target descriptionBCL2 interacting protein 3
- Target synonymsHABON, NIP3, BCL2/adenovirus E1B 19 kDa protein-interacting protein 3, BCL2/adenovirus E1B 19kDa interacting protein 3, BCL2/adenovirus E1B interacting protein 3, Hypoxia-Activated BNIP3 Overlapping Non-coding RNA, nineteen kD interacting protein-3
- HostMouse
- IsotypeIgG2b
- Protein IDQ12983
- Protein NameBCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- Scientific DescriptionBNIP3, formerly NIP3 (nineteen kDa interacting protein-3), is a pro-apoptotic, mitochondrial protein classified in the Bcl 2 family based on limited sequence homology to the Bcl 2 homology 3 (BH3) domain (amino acids 110-118) and C-terminal TM domain. BNIP3 expressed in yeast and mammalian cells interacts with survival promoting proteins Bcl 2, Bcl XL, CED9 and the adenovirus E1B 19K protein. Typically the BH3 domain of pro-apoptotic Bcl-2 homologues mediates Bcl 2/Bcl XL heterodimerization and confers pro-apoptotic activity. BNIP3 represents a subfamily of Bcl 2 related proteins, which functions without a typical BH3 domain to regulate apoptosis from both mitochondrial and nonmitochondrial sites by selective Bcl 2/Bcl XL interactions. The N-terminus (residues 1-49) and the C-terminus TM domain of BNIP3 are critical for Bcl 2 heterodimerization, and either region is sufficient for Bcl XL interaction. The TM domain of BNIP3 is critical for homodimerization, pro-apoptotic function, and mitochondrial targeting. BNIP3 contains PEST sequences suggesting that the protein may be susceptible to rapid degradation by proteases. PEST sequences commonly contain high local concentrations of amino acids P, E, S, T, and D flanked by charged amino acids and these are abundantly present in NIP3. Thus, the posttranslational control of BNIP3 expression through rapid protein degradation may constitute a mechanism for regulating the intracellular levels of a potentially lethal protein. Homologues of BNIP3 sharing both structural and functional similarity have been identified in mammals: Nix (also called BNIP3L/BNIP3alpha\/B5) and, in C. elegans, ceBNIP3. The TM domain of BNIP3 and Nix share 80% identity. Endogenous BNIP3 is loosely associated with mitochondrial membrane in normal tissue but fully integrates into the mitochondrial outer membrane with the N-terminus in the cytoplasm and the C-terminus in the membrane during induction of cell death. This is accompanied by rapid and profound mitochondrial dysfunction characterized by opening of the mitochondrial permeability transition (PT) pore, proton electrochemical gradient suppression, and increased reactive oxygen species production. BNIP3 has been reported to localize to the nuclear envelope when co-expressed with E1B 19K. Endogenous BNIP3 protein is abundant in murine andhuman skeletal muscle and is not detectable in lysates of all other nonskeletal muscle-bearing tissues and many cell lines, including myoblasts and differentiated myocytes.
- ReactivityHuman
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161
References
- Human antigen R regulates hypoxia-induced mitophagy in renal tubular cells through PARKIN/BNIP3L expressions. Yu SH et al., 2021 Mar, J Cell Mol MedRead this paper
- Gene expression profiling of bone marrow endothelial cells in patients with multiple myeloma. Ria R et al., 2009 Sep 1, Clin Cancer ResRead this paper