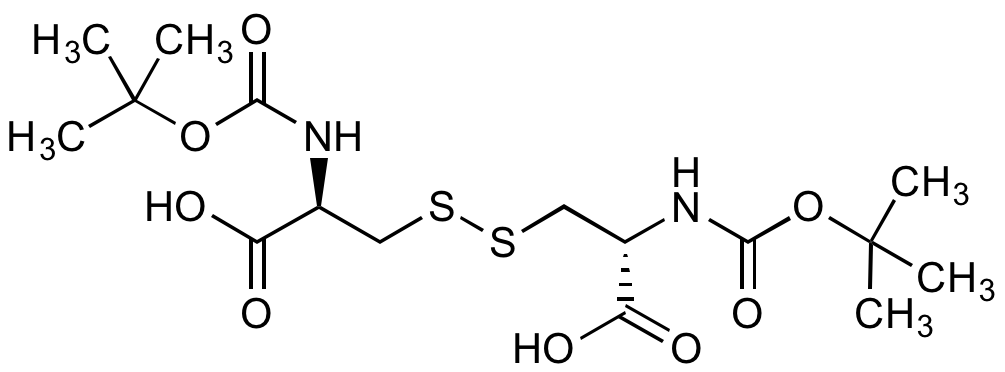
Chemical Structure
(Boc-Cys-OH)2 [10389-65-8] [10389-65-8]
CDX-B0224
CAS Number10389-65-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight440.53
Overview
- SupplierChemodex
- Product Name(Boc-Cys-OH)2 [10389-65-8] [10389-65-8]
- Delivery Days Customer2
- CAS Number10389-65-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC16H28N2O8S2
- Molecular Weight440.53
- Scientific DescriptionAmino acid protected by tert-butoxycarbonyl (Boc) group. Used as a reactant in peptide synthesis. Used to obtain asymmetric cystine peptides by enzymatic catalysis with immobilized papain. Used as educt for obtaining S-isoacyl dipeptide building blocks. - Chemical. CAS: 10389-65-8. Formula: C16H28N2O8S2. MW: 440.53. Synthetic. Amino acid protected by tert-butoxycarbonyl (Boc) group. Used as a reactant in peptide synthesis. Used to obtain asymmetric cystine peptides by enzymatic catalysis with immobilized papain. Used as educt for obtaining S-isoacyl dipeptide building blocks.
- SMILESCC(C)(C)OC(=O)N[C@@H](CSSC[C@H](NC(=O)OC(C)(C)C)C(O)=O)C(O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
