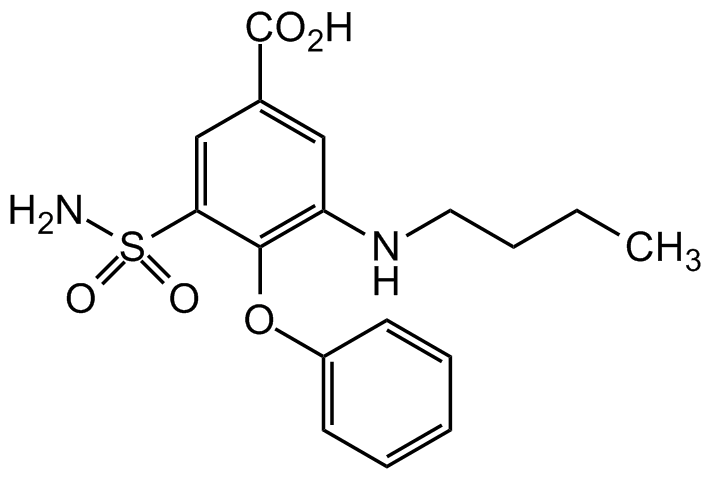
Chemical Structure
Bumetanide [28395-03-1] [28395-03-1]
AG-CR1-3543
CAS Number28395-03-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight364.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameBumetanide [28395-03-1] [28395-03-1]
- Delivery Days Customer10
- CAS Number28395-03-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC17H20N2O5S
- Molecular Weight364.4
- Scientific DescriptionBumetanide is a loop diuretic that is clinically used to treat congestive heart failure. Bumetanide also exhibits anxiolytic, neuroprotective and anti-epileptic/anticonvulsant activities. Bumetanide induces hyperpolarization in kidney cells, increasing whole-cell conductance and intracellular Ca2+ levels. Bumetanide acts by antagonizing Na+/K+/2Cl- symporter 1 (NKCC1), K+/Ca2+ co-transporter 2 (KCC2) and NKCC2, decreasing fluid secretion and Na+/K+ transport. While NKCC1 is expressed both in the CNS and in systemic organs, NKCC2 is kidney-specific. Bumetanide is a potent and specific inhibitor of Na-K-2Cl cotransporter 1 (NKCC1; IC50 = 0.68microM). It is selective for NKCC1 over NKCC2 (IC50 = 4microM). It is also an agonist of G protein-coupled receptor 35 (GPR35; EC50 = 10microM) and can inhibit carbonic anhydrases IX and XII. In physiological conditions, adult neurons have low intracellular Cl- levels underlying the gamma-aminobutyric acid (GABA)ergic inhibitory drive. Neurons have high (Cl-)I levels and excitatory GABA actions in a wide range of pathological conditions including spinal cord lesions, chronic pain, brain trauma, cerebrovascular infarcts, autism, Rett and Down syndrome, various types of epilepsies, and other genetic or environmental insults. By specifically inhibiting the chloride importer NKCC1, bumetanide efficiently restores low (Cl-)I levels and attenuates many disorders in experimental conditions. Bumetanide has been shown to improve electrophysiological, pathological and cognitive deficits in APOE4 knock-in mice. And single-nucleus RNA sequencing of neurons derived from APOE4 induced pluripotent stem cell (iPSC) showed transcriptomic reversal of Alzheimers disease (AD) signatures. - Chemical. CAS: 28395-03-1. Formula: C17H20N2O5S. MW: 364.4. Bumetanide is a loop diuretic that is clinically used to treat congestive heart failure. Bumetanide also exhibits anxiolytic, neuroprotective and anti-epileptic/anticonvulsant activities. Bumetanide induces hyperpolarization in kidney cells, increasing whole-cell conductance and intracellular Ca2+ levels. Bumetanide acts by antagonizing Na+/K+/2Cl- symporter 1 (NKCC1), K+/Ca2+ co-transporter 2 (KCC2) and NKCC2, decreasing fluid secretion and Na+/K+ transport. While NKCC1 is expressed both in the CNS and in systemic organs, NKCC2 is kidney-specific. Bumetanide is a potent and specific inhibitor of Na-K-2Cl cotransporter 1 (NKCC1; IC50 = 0.68microM). It is selective for NKCC1 over NKCC2 (IC50 = 4microM). It is also an agonist of G protein-coupled receptor 35 (GPR35; EC50 = 10microM) and can inhibit carbonic anhydrases IX and XII. In physiological conditions, adult neurons have low intracellular Cl- levels underlying the gamma-aminobutyric acid (GABA)ergic inhibitory drive. Neurons have high (Cl-)I levels and excitatory GABA actions in a wide range of pathological conditions including spinal cord lesions, chronic pain, brain trauma, cerebrovascular infarcts, autism, Rett and Down syndrome, various types of epilepsies, and other genetic or environmental insults. By specifically inhibiting the chloride importer NKCC1, bumetanide efficiently restores low (Cl-)I levels and attenuates many disorders in experimental conditions. Bumetanide has been shown to improve electrophysiological, pathological and cognitive deficits in APOE4 knock-in mice. And single-nucleus RNA sequencing of neurons derived from APOE4 induced pluripotent stem cell (iPSC) showed transcriptomic reversal of Alzheimers disease (AD) signatures.
- SMILESNS(C1=CC(C(O)=O)=CC(NCCCC)=C1OC2=CC=CC=C2)(=O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

