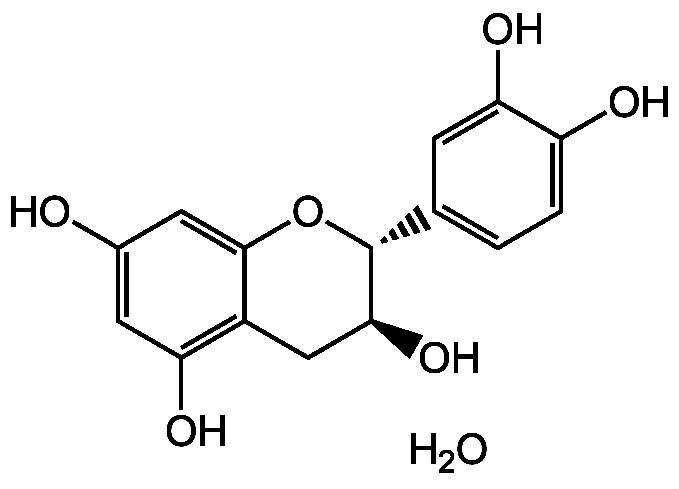
Chemical Structure
(+)-Catechin . hydrate [225937-10-0,88191-48-4] [225937-10-0]
AG-CN2-0407
CAS Number225937-10-0
Product group Chemicals
Estimated Purity>95%
Molecular Weight290.3 . 18.0
Overview
- SupplierAdipoGen Life Sciences
- Product Name(+)-Catechin . hydrate [225937-10-0,88191-48-4] [225937-10-0]
- Delivery Days Customer10
- CAS Number225937-10-0
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC15H14O6 . H2O
- Molecular Weight290.3 . 18.0
- Scientific DescriptionChemical. CAS: 225937-10-0 or 88191-48-4. Formula: C15H14O6 . H2O. MW: 290.3 . 18.0. Isolated from Uncaria rhynchophylla. Specific histidine decarboxylase inhibitor. Anti-inflammatory. COX-1 inhibitor. Antioxidant flavonoid. Free radical scavenger. Inhibits lipid peroxidation. Antimetastatic and anticancer compound. Antiangiogenic. Anti-osteoporotic. Antibacterial and antifungal. Neuroprotective. Mono oxidase B inhibitor. Apoptosis and cell cycle arrest inducer. JNK phosphorylation inhibitor. Antispasmodic. Insulin-mimetic. - Specific histidine decarboxylase inhibitor [1]. Anti-inflammatory. COX-1 inhibitor [2]. Antioxidant flavonoid. Free radical scavenger. Inhibits lipid peroxidation [3, 6, 9]. Antimetastatic and anticancer compound [4, 9, 10, 14, 16, 17]. Antiangiogenic [14]. Anti-osteoporotic [5]. Antibacterial and antifungal [7]. Neuroprotective [8]. Mono oxidase B inhibitor [11]. Apoptosis and cell cycle arrest inducer [9, 10, 16, 17]. JNK phosphorylation inhibitor [12]. Antispasmodic [13]. Insulin-mimetic [15].
- SMILESO.O[C@H]1CC2=C(O)C=C(O)C=C2O[C@@H]1C1=CC=C(O)C(O)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![(+)-Catechin Hydrate [225937-10-0] [225937-10-0]](https://www.targetmol.com/group3/M00/35/3F/CgoaEWayGjuELnmLAAAAAKEA-Wg689.png)