CLIA Kit for Bone Morphogenetic Protein 9 (BMP9)
SCB728HU
Product group Assays
Overview
- SupplierCloud-Clone Corp.
- Product NameCLIA Kit for Bone Morphogenetic Protein 9 (BMP9)
- Delivery Days Customer12
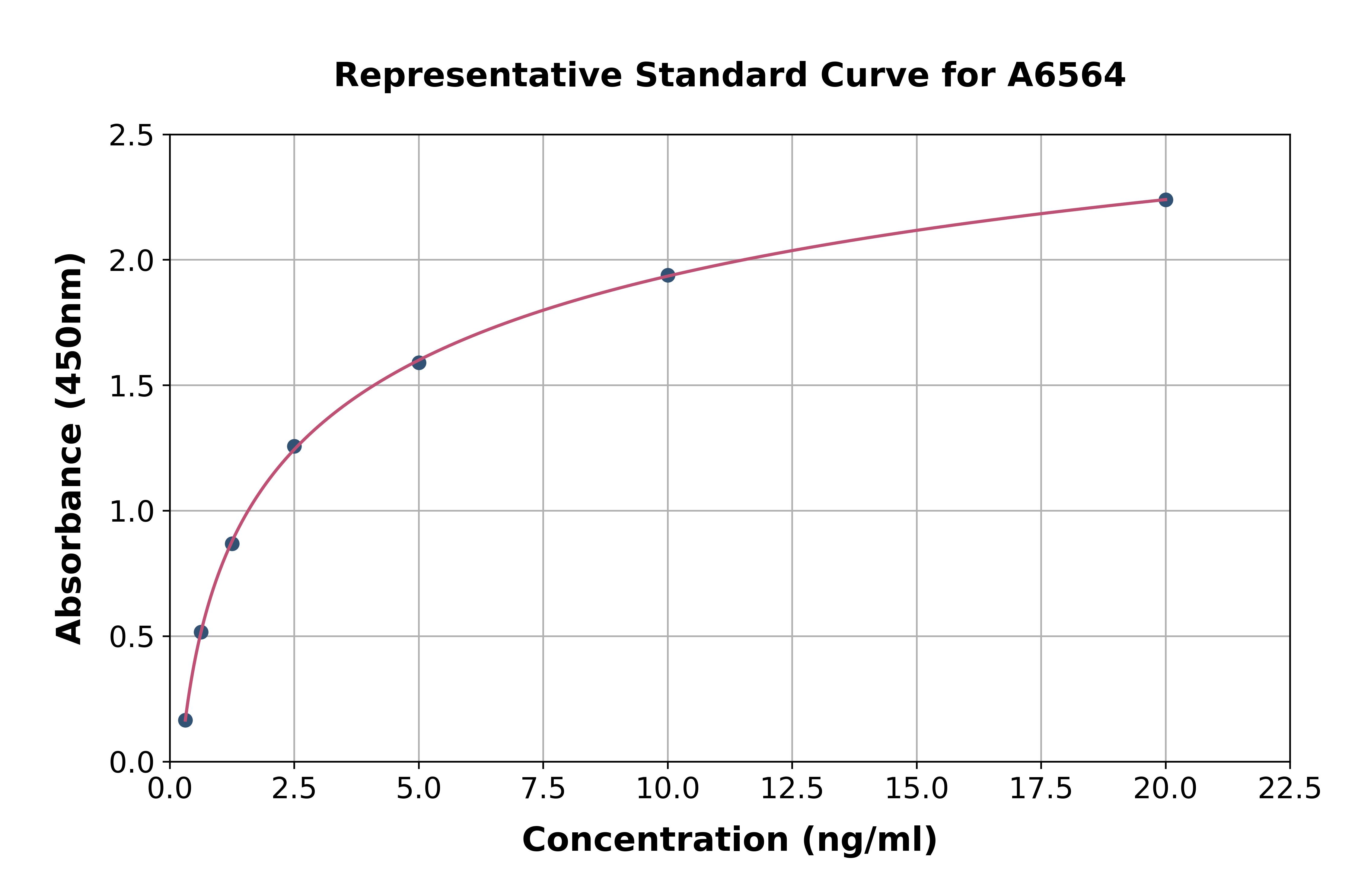
- Assay Detection Range68.6-50,000pg/mL
- Assay PrecisionIntra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Bone Morphogenetic Protein 9 (BMP9) were tested 20 times on one plate, respectively. Inter-assay Precision (Precision between assays): 3 samples with low, ...
- Assay SensitivityThe minimum detectable dose of this kit is typically less than 27.5pg/mL
- Assay Test PrincipleThe microplate provided in this kit has been pre-coated with an antibody specific to Bone Morphogenetic Protein 9 (BMP9). Standards or samples are then added to the appropriate microplate wells with a biotin-conjugated antibody specific to Bone ...
- Assay Time2h, 40min
- CertificationResearch Use Only
- UNSPSC41105332