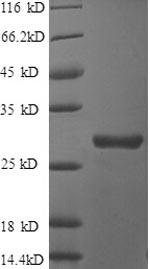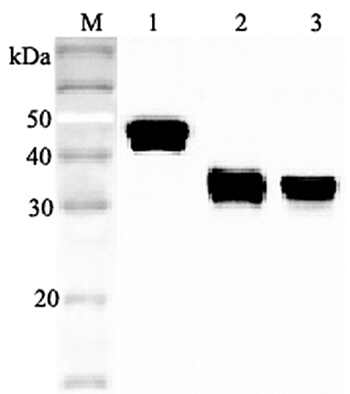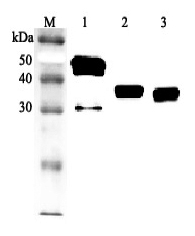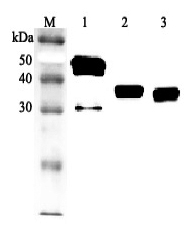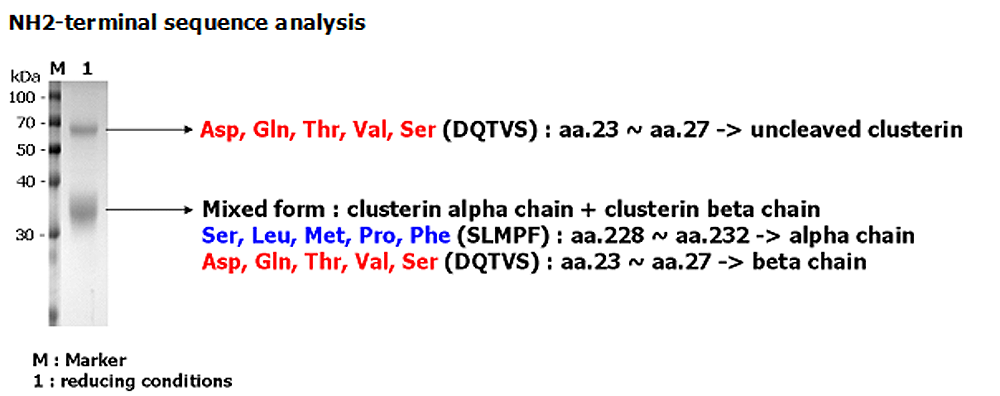
After denaturation of recombinant secretory clusterin in the presense of beta-mercaptoethanol followed by boiling, two distinct bands, 60kDa and 35kDa, were seperated on SDS-PAGE. The two bands were extracted and subjected to determination of their N-te
Clusterin (secretory form) (human) (rec.)
AG-40A-0050Y
Protein IDP10909
Product group Proteins / Signaling Molecules
Overview
- SupplierAdipoGen Life Sciences
- Product NameClusterin (secretory form) (human) (rec.)
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>90%
- Gene ID1191
- Target nameCLU
- Target descriptionclusterin
- Target synonymsAAG4, APO-J, APOJ, CLI, CLU1, CLU2, KUB1, NA1/NA2, SGP-2, SGP2, SP-40, TRPM-2, TRPM2, clusterin, aging-associated protein 4, apolipoprotein J, complement cytolysis inhibitor, complement lysis inhibitor, complement-associated protein SP-40,40, epididymis secretory sperm binding protein, ku70-binding protein 1, sulfated glycoprotein 2, testosterone-repressed prostate message 2
- Protein IDP10909
- Protein NameClusterin
- Scientific DescriptionClusterin shares homology with the small heat shock protein family of molecular chaperones. The mature secreted form of the protein is a glycosylated, 80kDa disulfide-linked heterodimer of alpha and beta subunits (produced by internal cleavage). Clusterin is expressed in virtually all tissues and found in all human fluids. It is involved in numerous physiological processes important for carcinogenesis and tumor growth, including apoptotic cell death, cell cycle regulation, DNA repair, cell adhesion, tissue remodeling, lipid transportation, membrane recycling and immune system regulation. Clusterin also exists as a nuclear protein. The secreted form of Clusterin has extracellular chaperone and anti-apoptotic activities while the nuclear form acts as a proapoptotic factor. - Protein. Signal peptide sequence and the human secretory clusterin (aa 1-449) are fused at the C-terminus to a FLAG®-tag. Source: HEK 293 cells. Endotoxin content: 90% (SDS-PAGE). Clusterin shares homology with the small heat shock protein family of molecular chaperones. The mature secreted form of the protein is a glycosylated, 80kDa disulfide-linked heterodimer of alpha and beta subunits (produced by internal cleavage). Clusterin is expressed in virtually all tissues and found in all human fluids. It is involved in numerous physiological processes important for carcinogenesis and tumor growth, including apoptotic cell death, cell cycle regulation, DNA repair, cell adhesion, tissue remodeling, lipid transportation, membrane recycling and immune system regulation. Clusterin also exists as a nuclear protein. The secreted form of Clusterin has extracellular chaperone and anti-apoptotic activities while the nuclear form acts as a proapoptotic factor.
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116100
- SpeciesHuman