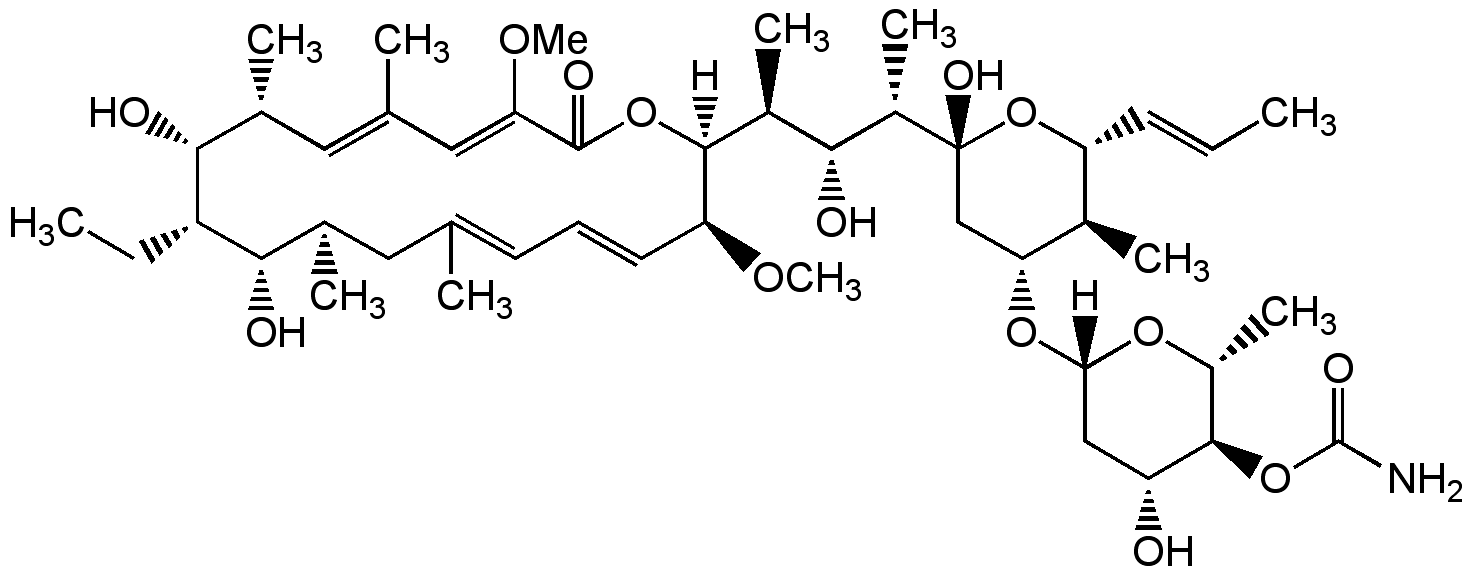
Chemical Structure
Concanamycin A (high purity) [80890-47-7] [80890-47-7]
BVT-0237
CAS Number80890-47-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight866.1
Overview
- SupplierBioViotica
- Product NameConcanamycin A (high purity) [80890-47-7] [80890-47-7]
- Delivery Days Customer2
- CAS Number80890-47-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC46H75NO14
- Molecular Weight866.1
- Scientific DescriptionAntibiotic. More potent and specific H+-ATPase inhibitor than bafilomycin A1 (Prod. No. BVT-0252). Inhibits acidification of organelles such as lysosomes and the Golgi apparatus. Inhibitor of autophagic degradation by rising lysosomal pH and thus inactivating the lysosomal acid hydrolases. Blocks cell surface expression of viral glycoproteins without affecting their synthesis. Cytotoxic in a number of cell lines in a cell viability assay. Induces nitric oxide (NO) production. Concanamycin A enables the immune system to kill HIV-infected cells. Nef is an HIV-encoded accessory protein that enhances pathogenicity by down-regulating major histocompatibility class I (MHC-I) expression to evade killing by cytotoxic T lymphocytes (CTLs). Concanamycin A has been identified as potent inhibitor of Nef that restores MHC-I and enhances the clearance of HIV-infected primary cells by cytotoxic T lymphocytes. Concanamycin A counteracts Nef from diverse clades of HIV targeting multiple allotypes of MHC-I, indicating the potential for broad therapeutic utility. - Chemical. CAS: 80890-47-7. Formula: C46H75NO14. MW: 866.1. Isolated from Streptomyces sp. Antibiotic. More potent and specific H+-ATPase inhibitor than bafilomycin A1 (Prod. No. BVT-0252). Inhibits acidification of organelles such as lysosomes and the Golgi apparatus. Inhibitor of autophagic degradation by rising lysosomal pH and thus inactivating the lysosomal acid hydrolases. Blocks cell surface expression of viral glycoproteins without affecting their synthesis. Cytotoxic in a number of cell lines in a cell viability assay. Induces nitric oxide (NO) production.
- SMILES[H][C@@]1(C[C@@H](O)[C@H](OC(N)=O)[C@@H](C)O1)O[C@@H]1C[C@@](O)(O[C@H](\C=C\C)[C@H]1C)[C@@H](C)[C@H](O)[C@H](C)[C@]1([H])OC(=O)\C(OC)=C\C(\C)=C\[C@@H](C)[C@@H](O)[C@@H](CC)[C@@H](O)[C@H](C)CC(C)=C\C=C\[C@@H]1OC
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200


![Concanamycin A [80890-47-7] [80890-47-7]](https://www.targetmol.com/group3/M00/36/C5/CgoaEGayQ2KEck4vAAAAAIXg0tI628.png)