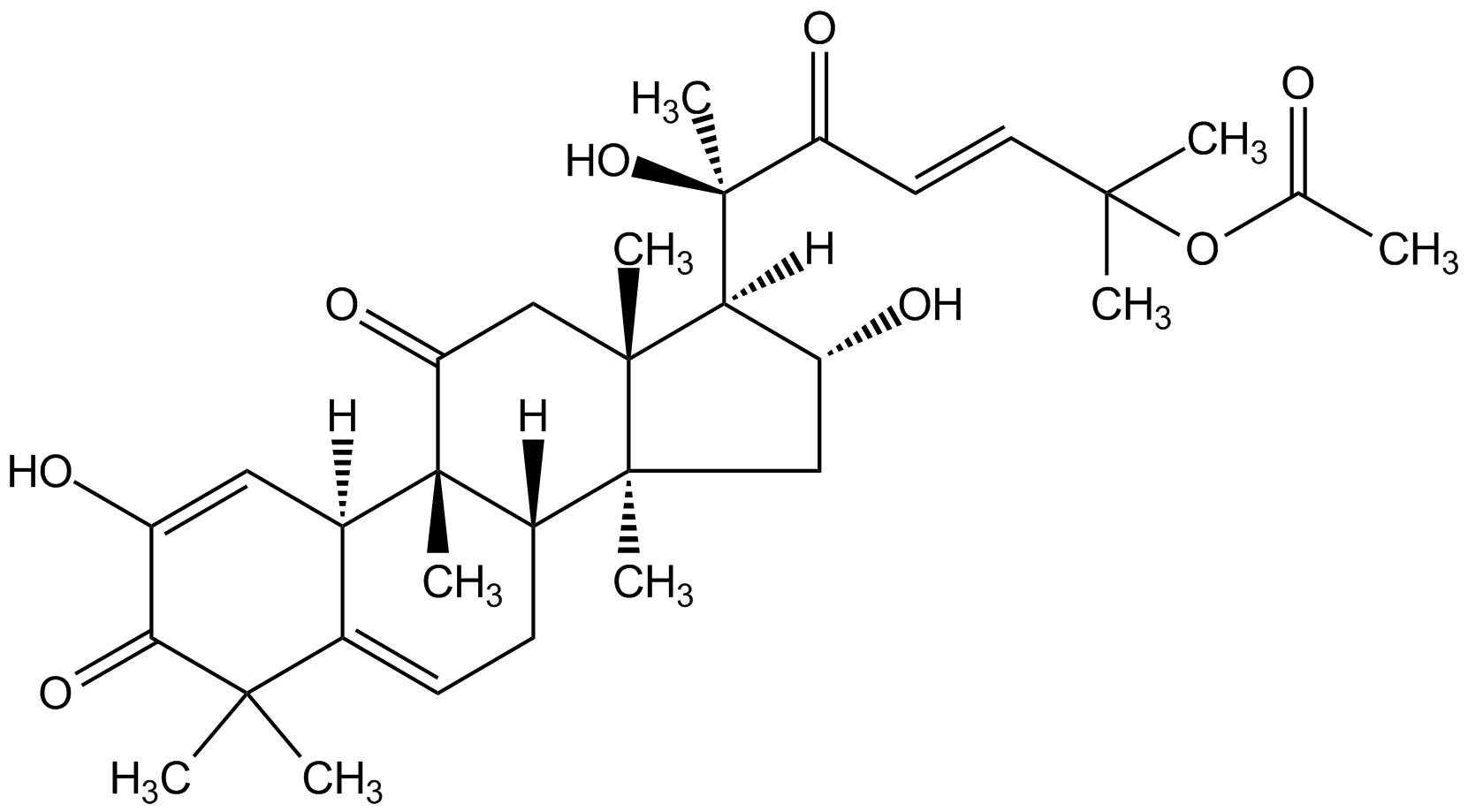
Chemical Structure
Cucurbitacin E [18444-66-1] [18444-66-1]
AG-CN2-0474
CAS Number18444-66-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight556.7
Overview
- SupplierAdipoGen Life Sciences
- Product NameCucurbitacin E [18444-66-1] [18444-66-1]
- Delivery Days Customer10
- CAS Number18444-66-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC32H44O8
- Molecular Weight556.7
- Scientific DescriptionChemical. CAS: 18444-66-1. Formula: C32H44O8. MW: 556.7. Isolated from Cucumis melo L. Potent actin depolymerization inhibitor. Shown to have a different mechanism of action compared to jasplakinolide (Prod. No. AG-CN2-0037), binding to a different site. Binds specifically to filamentous actin (F-actin) forming a covalent bond at residue Cys257, stabilizing F-actin without affecting actin polymerization or nucleation. Does not bind to monomeric actin (G-actin). Immunomodulator with anti-inflammatory and anti-tumorigenic properties in a range of cancer cell lines, mediated by its action on the cellular cytoskeleton, on mitotic pathways as well as on cellular autophagy. Shown to induce apoptosis, autophagy and cell cycle arrest at G2/M. Inhibits tumor angiogenesis through Jak-STAT3 signaling pathway, suppresses cell migration and invasion, and inhibits NF-kappaB nuclear translocation. Antioxidant and potential neuroprotective compound with potential neurodegenerative properties. - Potent actin depolymerization inhibitor. Shown to have a different mechanism of action compared to jasplakinolide (Prod. No. AG-CN2-0037), binding to a different site. Binds specifically to filamentous actin (F-actin) forming a covalent bond at residue Cys257, stabilizing F-actin without affecting actin polymerization or nucleation. Does not bind to monomeric actin (G-actin). Immunomodulator with anti-inflammatory and anti-tumorigenic properties in a range of cancer cell lines, mediated by its action on the cellular cytoskeleton, on mitotic pathways as well as on cellular autophagy. Shown to induce apoptosis, autophagy and cell cycle arrest at G2/M. Inhibits tumor angiogenesis through Jak-STAT3 signaling pathway, suppresses cell migration and invasion, and inhibits NF-kappaB nuclear translocation. Antioxidant and potential neuroprotective compound with potential neurodegenerative properties.
- SMILES[H][C@@]1([C@H](O)C[C@@]2(C)[C@]3([H])CC=C4[C@@]([H])(C=C(O)C(=O)C4(C)C)[C@]3(C)C(=O)C[C@]12C)[C@@](C)(O)C(=O)\C=C\C(C)(C)OC(C)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Cucurbitacin E [18444-66-1] [18444-66-1]](https://www.targetmol.com/group3/M00/35/79/CgoaEWayIP2EWjqZAAAAAEQ4_mc409.png)