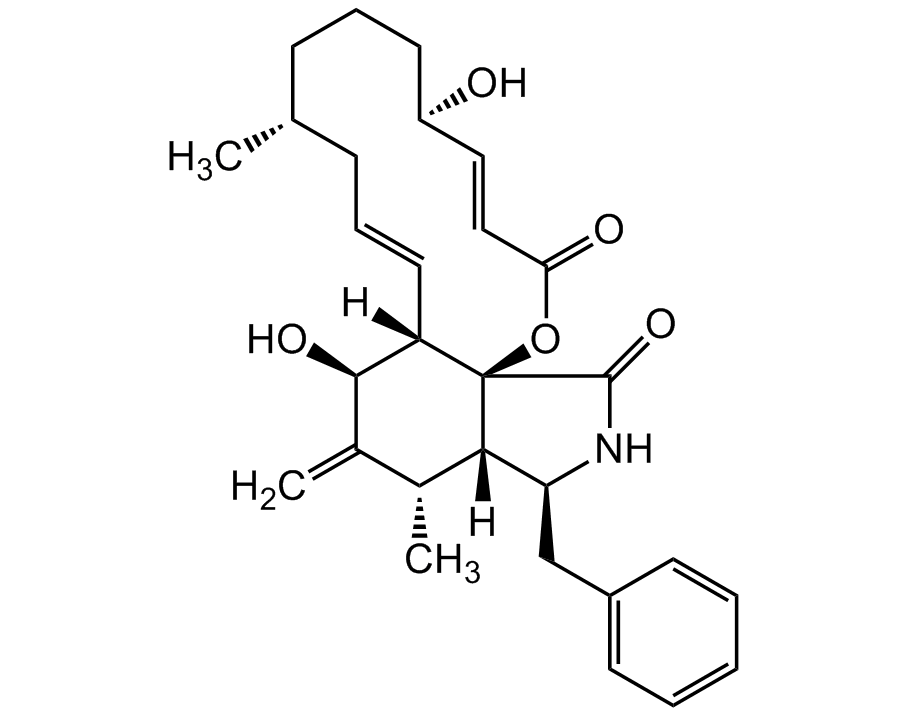
Chemical Structure
Cytochalasin B [14930-96-2] [14930-96-2]
AG-CN2-0504
CAS Number14930-96-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight479.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameCytochalasin B [14930-96-2] [14930-96-2]
- Delivery Days Customer10
- CAS Number14930-96-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC29H37NO5
- Molecular Weight479.6
- Scientific DescriptionCell permeable mycotoxin. Actin polymerization inhibitor. Binds to the barbed end of actin, reversibly inhibiting the elongation and shortening of actin filaments. Induces nuclear extrusion. By disrupting actin polymerization, blocks diverse cellular functions, including cell division, migration, phagocytosis, exocytosis and chemotaxis. Induces DNA fragmentation. Used in cytoskeletal reorganization, cell imaging and organelle trafficking studies. Potent antitumor activity. Causes cell cycle arrest and induces apoptosis in cancer cells. Shown to have an inhibitory effect on tumor cell growth without causing immunosuppressive effects. Non-competitive glucose transport inhibitor. Inhibits of GLUT1/GLUT2 mediated monosaccharide transport across the plasma membrane. Does not inhibit GLUT5. Used for testing the genotoxicity of substances in the cytokinesis-block micronucleus cytome (CBMN cyt) assay. Used in cloning through nuclear transfer. Enucleated recipient cells are treated with cytochalasin B. Cytochalasin B makes the cytoplasm of the oocytes more fluid and makes it possible to aspirate the nuclear genome of the oocyte within a small vesicle of plasma membrane into a micro-needle. Decreases the expression levels of DNMT1, DNMT3a, DNMT3b, HAT1 and HDAC1 at the pronuclear stage in porcine embryos. Phosphatidylcholine and phosphatidylethanolamine biosynthesis inhibitor. Platelet aggregation inhibitor. - Chemical. CAS: 14930-96-2. Formula: C29H37NO5. MW: 479.6. Isolated from Phoma sp. Cell permeable mycotoxin. Actin polymerization inhibitor. Binds to the barbed end of actin, reversibly inhibiting the elongation and shortening of actin filaments. Induces nuclear extrusion. By disrupting actin polymerization, blocks diverse cellular functions, including cell division, migration, phagocytosis, exocytosis and chemotaxis. Induces DNA fragmentation. Used in cytoskeletal reorganization, cell imaging and organelle trafficking studies. Potent antitumor activity. Causes cell cycle arrest and induces apoptosis in cancer cells. Shown to have an inhibitory effect on tumor cell growth without causing immunosuppressive effects. Non-competitive glucose transport inhibitor. Inhibits of GLUT1/GLUT2 mediated monosaccharide transport across the plasma membrane. Does not inhibit GLUT5. Used for testing the genotoxicity of substances in the cytokinesis-block micronucleus cytome (CBMN cyt) assay. Used in cloning through nuclear transfer. Enucleated recipient cells are treated with cytochalasin B. Cytochalasin B makes the cytoplasm of the oocytes more fluid and makes it possible to aspirate the nuclear genome of the oocyte within a small vesicle of plasma membrane into a micro-needle. Decreases the expression levels of DNMT1, DNMT3a, DNMT3b, HAT1 and HDAC1 at the pronuclear stage in porcine embryos. Phosphatidylcholine and phosphatidylethanolamine biosynthesis inhibitor. Platelet aggregation inhibitor.
- SMILESC[C@@H]1C([C@@H](O)[C@@]2([H])[C@@]3(OC(/C=C/[C@H](O)CCC[C@@H](C)C/C=C/2)=O)[C@]1([H])[C@H](CC4=CC=CC=C4)NC3=O)=C
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 1544
- UNSPSC12352200


![Cytochalasin B [14930-96-2]](https://www.targetmol.com/group3/M00/36/C7/CgoaEGayQ36EeLZqAAAAAKFfZEw732.png)