Chemical Structure
DPM [143503-03-1] [143503-03-1]
CDX-D0049
CAS Number143503-03-1
Product group Chemicals
Estimated Purity>97%
Molecular Weight364.35
Overview
- SupplierChemodex
- Product NameDPM [143503-03-1] [143503-03-1]
- Delivery Days Customer2
- CAS Number143503-03-1
- CertificationResearch Use Only
- Estimated Purity>97%
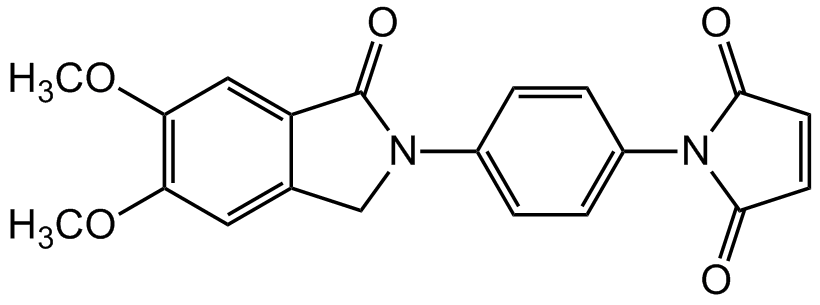
- Molecular FormulaC20H16N2O5
- Molecular Weight364.35
- Scientific DescriptionChemical. CAS: 143503-03-1. Formula: C20H16N2O5. MW: 364.35. Synthetic. Nonfluorescent N-substituted maleimides react selectively with thiol compounds to form fluorescent derivatives. Fluorescent intensity of the dimethoxyphthalimidyl group is higher than that of the nonsubsituted phthalimidyl group. The analytical sensitivity of N-[4-(5,6-Dimethoxy-N-phthalimidyl)phenyl]maleimide (DPM) is about 3-times higher than that of N-[4-(2-phthalimidyl)phenyl]maleimide (PPM). DPM can be used as a precolumn labeling reagent in HPLC. DPM was also used for the determination of physiologically important thiols in biological fluids or therapeutic drug monitoring. - Nonfluorescent N-substituted maleimides react selectively with thiol compounds to form fluorescent derivatives. Fluorescent intensity of the dimethoxyphthalimidyl group is higher than that of the nonsubsituted phthalimidyl group. The analytical sensitivity of N-[4-(5,6-Dimethoxy-N-phthalimidyl)phenyl]maleimide (DPM) is about 3-times higher than that of N-[4-(2-phthalimidyl)phenyl]maleimide (PPM). DPM can be used as a precolumn labeling reagent in HPLC. DPM was also used for the determination of physiologically important thiols in biological fluids or therapeutic drug monitoring.
- SMILESCOC1=C(OC)C=C2C(CN(C2=O)C2=CC=C(C=C2)N2C(=O)C=CC2=O)=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12162000

![N-4-(5,6-Dimethoxy-N-phthalimidinyl)phenylmaleimide [143503-03-1] [143503-03-1]](https://www.targetmol.com/group3/M00/38/2C/CgoaEWa8jySEML8CAAAAAJbDIK4243.png)