ELISA Kit for Dihydrotestosterone (DHT)
CEA443GE
Product group Assays
Overview
- SupplierCloud-Clone Corp.
- Product NameELISA Kit for Dihydrotestosterone (DHT)
- Delivery Days Customer12
- ApplicationsELISA
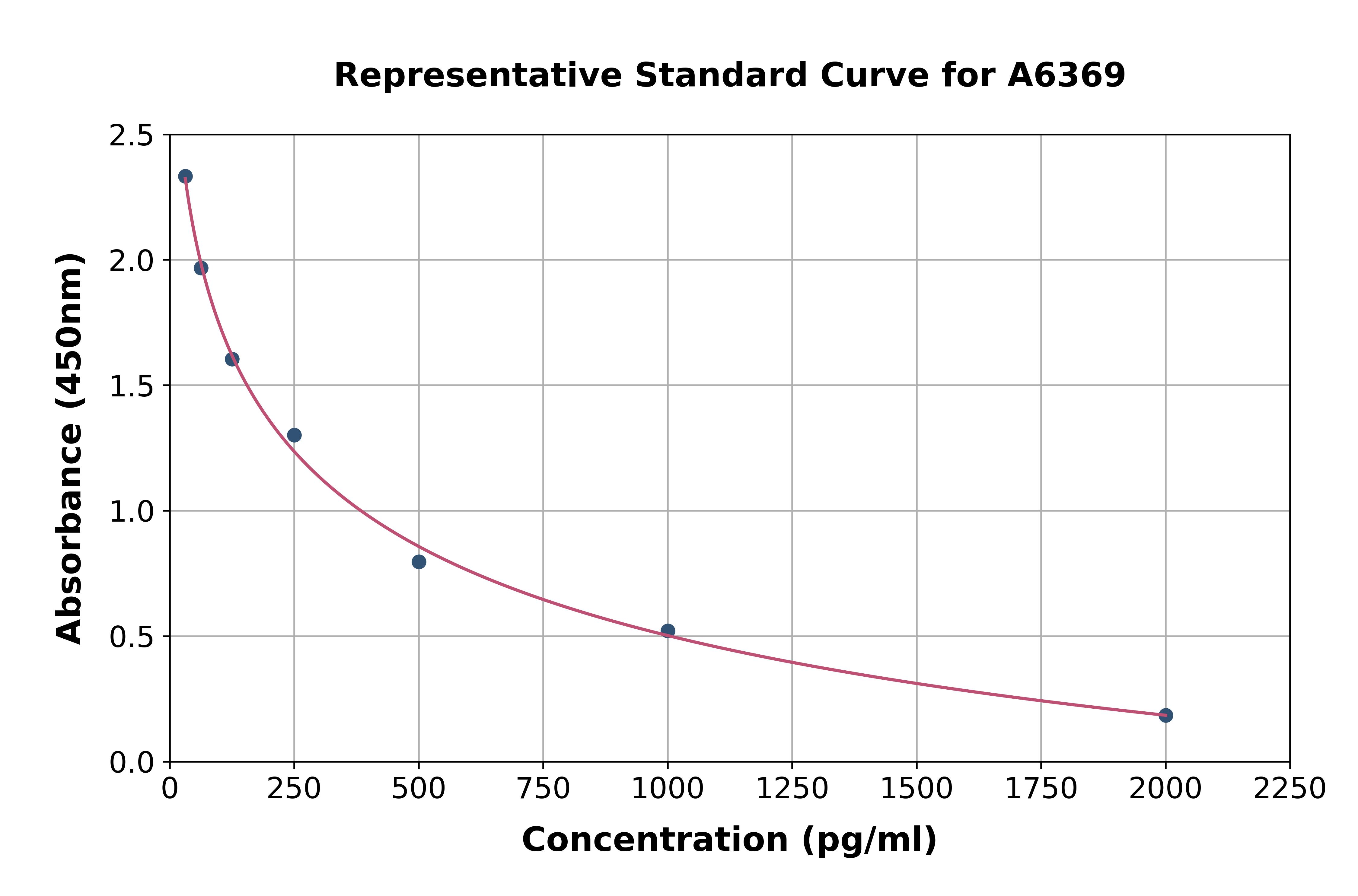
- Assay Detection Range30.9-2,500pg/mL
- Assay PrecisionIntra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Dihydrotestosterone (DHT) were tested 20 times on one plate, respectively. Inter-assay Precision (Precision between assays): 3 samples with low, middle and...
- Assay SensitivityThe minimum detectable dose of this kit is typically less than 10.7pg/mL
- Assay Test PrincipleThis assay employs the competitive inhibition enzyme immunoassay technique. A monoclonal antibody specific to Dihydrotestosterone (DHT) has been pre-coated onto a microplate. A competitive inhibition reaction is launched between biotin labeled...
- Assay Time2h
- CertificationResearch Use Only
- UNSPSC41116100
References
- Dihydrotestosterone Treatment Accelerates Autograft Reversal Sciatic Nerve Regeneration in Rats. Yang X et al., 2018 Mar, Neurochem ResRead this paper
- Testosterone Replacement Modulates Cardiac Metabolic Remodeling after Myocardial Infarction by Upregulating PPARalpha. Yang J et al., 2016, PPAR ResRead this paper
- Oral administration of low-dose bisphenol A promotes proliferation of ventral prostate and upregulates prostaglandin D(2) synthase expression in adult rats. Wu J et al., 2016 Nov, Toxicol Ind HealthRead this paper
- Treatment of benign prostatic hyperplasia with Croton membranaceus in an experimental animal model. Afriyie DK et al., 2014 Nov 18, J EthnopharmacolRead this paper