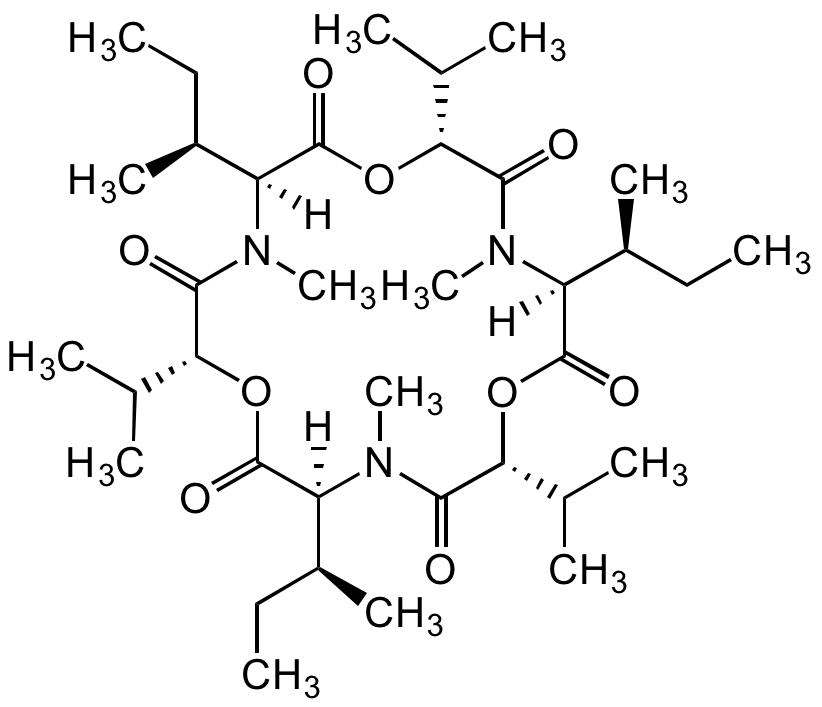
Chemical Structure
Enniatin A [2503-13-1] [2503-13-1]
AG-CN2-0477
CAS Number2503-13-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight681.9
Overview
- SupplierAdipoGen Life Sciences
- Product NameEnniatin A [2503-13-1] [2503-13-1]
- Delivery Days Customer10
- CAS Number2503-13-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC36H63N3O9
- Molecular Weight681.9
- Scientific DescriptionChemical. CAS: 2503-13-1. Formula: C36H63N3O9. MW: 681.9. Isolated from fungus Fusarium sp. Cyclohexadepsipeptide mycotoxin. One of four major analogs in the enniatin complex. Commonly found food contaminant in cereals and their products. Ionophore antibiotic. Incorporation into the cell membrane forms dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. Anticancer compound. Triggers apoptosis in several cancer cell lines. Leads to loss of mitochondrial membrane potential and cell cycle arrest. Moderate inhibitor of ACAT (acylcoenzyme A:cholesterolacyl transferase). Shown to have a variety of other biological activities such as antifungal, anthelmintic, insecticidal, immunomodulatory and phytotoxic activity. - Cyclohexadepsipeptide mycotoxin. One of four major analogs in the enniatin complex. Commonly found food contaminant in cereals and their products. Ionophore antibiotic. Incorporation into the cell membrane forms dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. Anticancer compound. Triggers apoptosis in several cancer cell lines. Leads to loss of mitochondrial membrane potential and cell cycle arrest. Moderate inhibitor of ACAT (acylcoenzyme A:cholesterolacyl transferase). Shown to have a variety of other biological activities such as antifungal, anthelmintic, insecticidal, immunomodulatory and phytotoxic activity.
- SMILES[H][C@@]1([C@@H](C)CC)N(C)C(=O)[C@H](OC(=O)[C@]([H])([C@@H](C)CC)N(C)C(=O)[C@H](OC(=O)[C@]([H])([C@@H](C)CC)N(C)C(=O)[C@H](OC1=O)C(C)C)C(C)C)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Enniatin A [2503-13-1] [2503-13-1]](https://www.targetmol.com/group3/M00/02/AE/CgoaEGY7OqSEOtWKAAAAADyTz0g568.png)