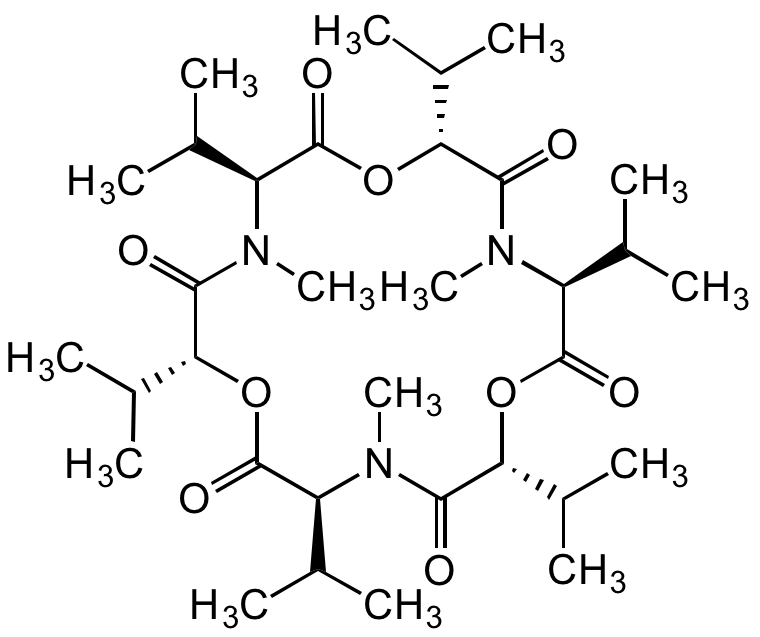
Chemical Structure
Enniatin B [917-13-5]
AG-CN2-0479
CAS Number917-13-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight639.8
Overview
- SupplierAdipoGen Life Sciences
- Product NameEnniatin B [917-13-5]
- Delivery Days Customer10
- CAS Number917-13-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC33H57N3O9
- Molecular Weight639.8
- Scientific DescriptionChemical. CAS: 917-13-5. Formula: C33H57N3O9. MW: 639.8. Isolated from fungus Fusarium sp. Cyclohexadepsipeptide mycotoxin. One of four major analogs in the enniatin complex. Commonly found food contaminant in cereals and their products. Ionophore antibiotic (less ionophoric than Enniatin A). Incorporation into the cell membrane forms dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. Anticancer compound. Triggers apoptosis in several cancer cell lines. Moderate inhibitor of ACAT (acylcoenzyme A:cholesterolacyl transferase). Inhibitor of the pleiotropic drug resistance protein 5 (Pdr5p) in yeast. Shown to have a variety of other biological activities such as antifungal, anthelmintic, insecticidal, immunomodulatory and phytotoxic activity. - Cyclohexadepsipeptide mycotoxin. One of four major analogs in the enniatin complex. Commonly found food contaminant in cereals and their products. Ionophore antibiotic (less ionophoric than Enniatin A). Incorporation into the cell membrane forms dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling. Anticancer compound. Triggers apoptosis in several cancer cell lines. Moderate inhibitor of ACAT (acylcoenzyme A:cholesterolacyl transferase). Inhibitor of the pleiotropic drug resistance protein 5 (Pdr5p) in yeast. Shown to have a variety of other biological activities such as antifungal, anthelmintic, insecticidal, immunomodulatory and phytotoxic activity.
- SMILESCC(C)[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@H](C(C)C)N(C)C1=O)C(C)C)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Enniatin B [917-13-5]](https://www.targetmol.com/group3/M00/36/C3/CgoaEWayQ32EQb4aAAAAAMitt1M920.png)