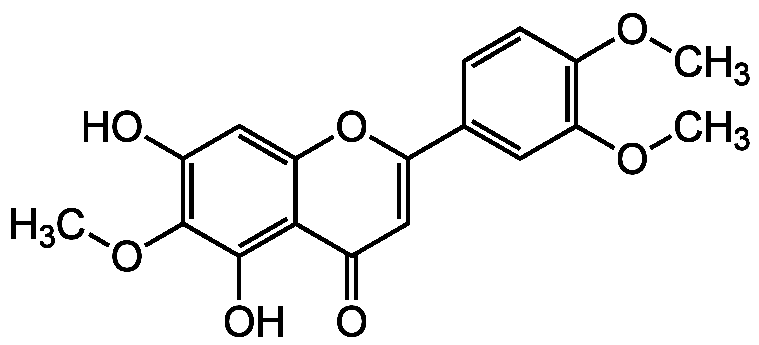
Chemical Structure
Eupatilin [22368-21-4] [22368-21-4]
AG-CN2-0432
CAS Number22368-21-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight344.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameEupatilin [22368-21-4] [22368-21-4]
- Delivery Days Customer10
- CAS Number22368-21-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC18H16O7
- Molecular Weight344.3
- Scientific DescriptionChemical. CAS: 22368-21-4. Formula: C18H16O7. MW: 344.3. Isolated from Artemisia sp. Selective inhibitor of 5-lipoxygenase. Apoptosis and cell cycle arrest inducer. Anticancer compound. Inhibits ERK1/2, JNK, and NF-kappaB activation and expression of Raf-1 and Ras. Anti-proliferative compound. Anti-inflammatory and immunosuppressive. Anti-apoptotic compound in hepatocytes. Antioxidant. Antidiabetic. Enhances hepatic and plasma glucose metabolism and increases insulin secretion in type 2 diabetic mice. Inhibits angiogenesis by blocking STAT3 and VEGF expression. Neuroprotective. PI3K Class I, MKK3/6 and MKK4 inhibitor. Shown to inhibit osteoporosis dually through transcriptional suppression and actin rearrangement. - Selective inhibitor of 5-lipoxygenase. Anti-proliferative and anticancer compound. Apoptosis and cell cycle arrest inducer. Inhibits ERK1/2, JNK, and NF-kappaB activation and expression of Raf-1 and Ras. PI3K Class I, MKK3/6 and MKK4 inhibitor. Inhibits angiogenesis by blocking STAT3 and VEGF expression. Anti-inflammatory and immunosuppressive agent. Antioxidant. Antidiabetic. Enhances hepatic and plasma glucose metabolism and increases insulin secretion in type 2 diabetic mice. Selective PPARalpha agonist. Neuroprotective.
- SMILESCOC1=C(C=C(C=C1)C2=CC(=O)C3=C(C(=C(C=C3O2)O)OC)O)OC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Eupatilin [22368-21-4] [22368-21-4]](https://www.targetmol.com/group3/M00/35/62/CgoaEGayHY-EDfBMAAAAAAI32x4257.png)