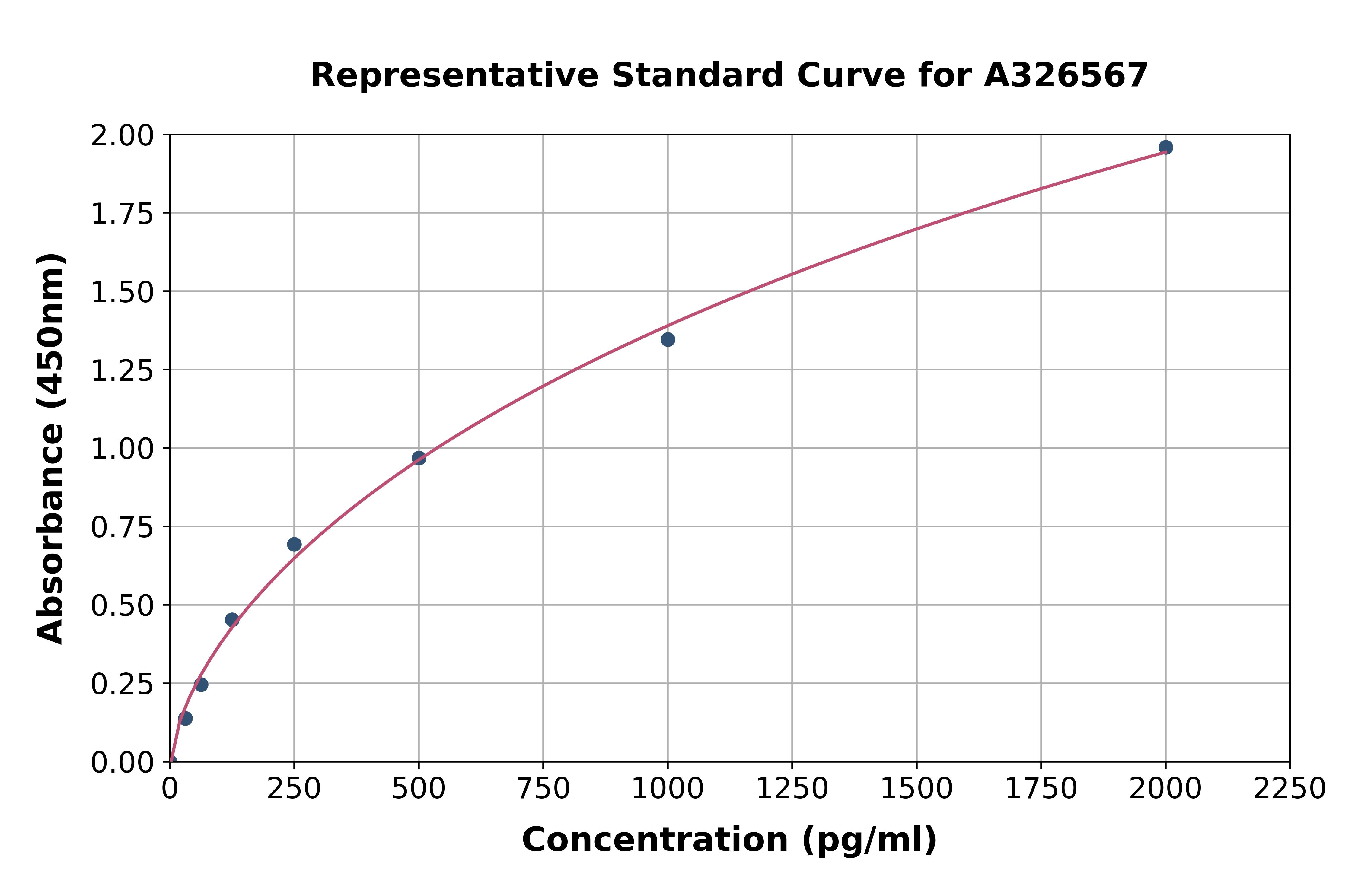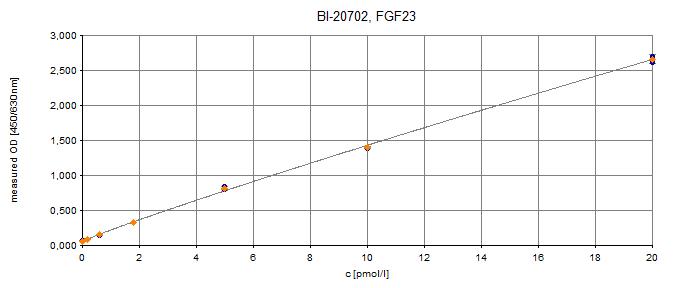
The figure shows a typical standard curve for the human C-terminal FGF23 ELISA. The immunoassay is calibrated against recombinant C-terminal FGF23 peptide.
FGF23 (C-terminal) ELISA
BI-20702
Assay Sample Typeserum, EDTA plasma, citrate plasma, heparin plasma
Product group Assays
Overview
- SupplierBiomedica
- Product NameFGF23 (C-terminal) ELISA
- Delivery Days Customer7
- ApplicationsELISA
- Applications SupplierELISA
- Assay Detection Range0.2-20 pmol/l
- Assay Precisionintra-assay: 12%, inter-assay: 10%
- Assay Sample Typeserum, EDTA plasma, citrate plasma, heparin plasma
- Assay Sensitivity0.08 pmol/l
- Assay Test PrincipleSandwich ELISA
- Assay Timeovernight assay
- CertificationResearch Use Only
- Scientific DescriptionProduct Characteristics: The Biomedica human C-terminal FGF23 (fibroblast-growth factor 23) Sandwich ELISA kit is intended for the quantitative measurement of FGF23 levels serum and plama samples (EDTA, heparin and citrate). The human C-terminal FGF23 ELISA is internationally recognised with >55 FGF23 ELISA specific references to date. Target Information: FGF23 (fibroblast growth factor 23) is a 32-kDa protein with 251 amino acids that is proteolytically processed between arginine179 and serine180 to generate N-terminal and C-terminal fragments. FGF23 is mainly secreted by osteocytes and controls phosphate and 1,25(OH)2 vitamin D homeostasis.
- Storage Instruction2-8°C
- UNSPSC41116133