FGL1 (human) ELISA Kit
AG-45B-0022
ReactivityHuman
Product group Assays
Overview
- SupplierAdipoGen Life Sciences
- Product NameFGL1 (human) ELISA Kit
- Delivery Days Customer10
- ApplicationsELISA
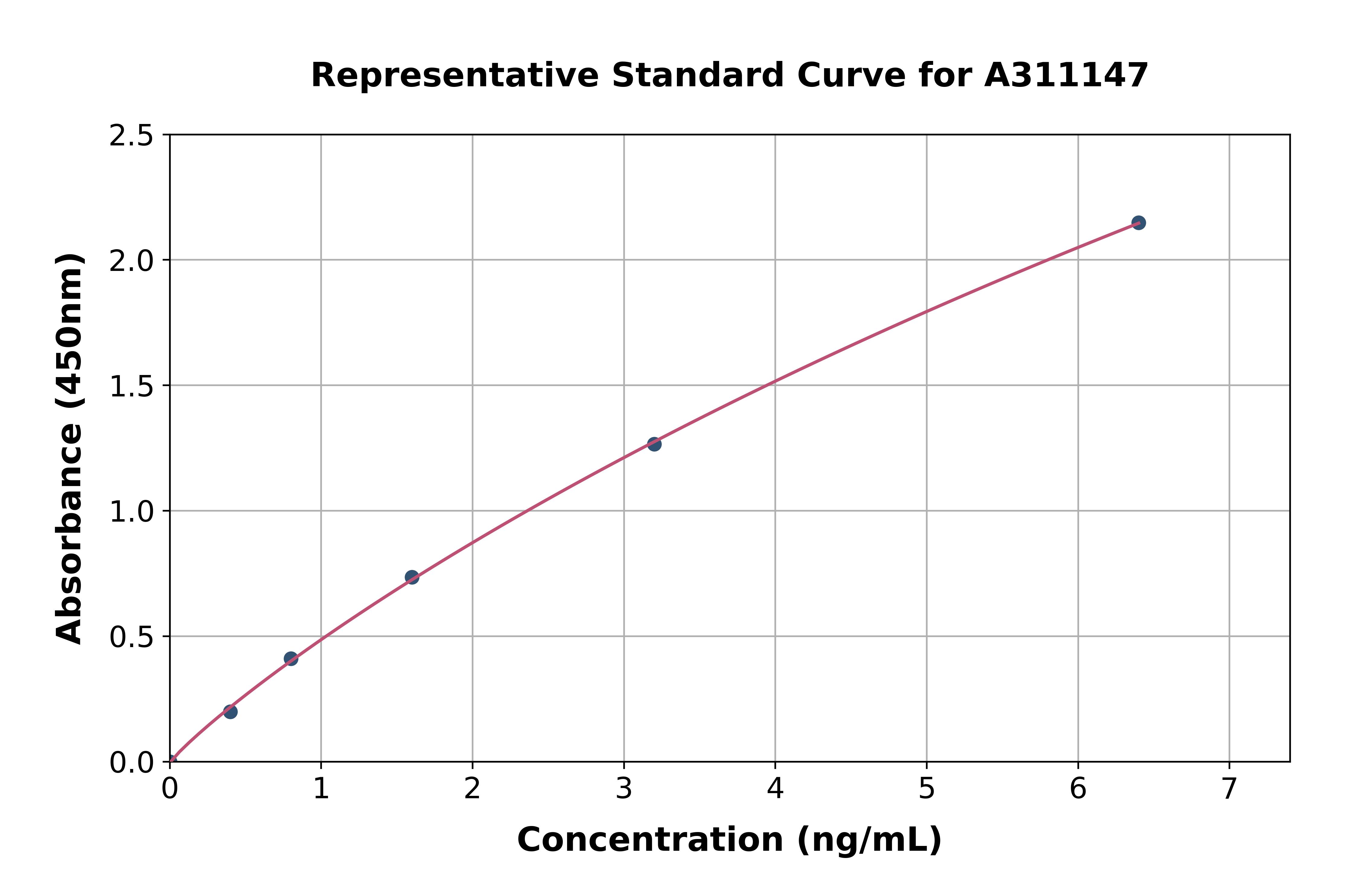
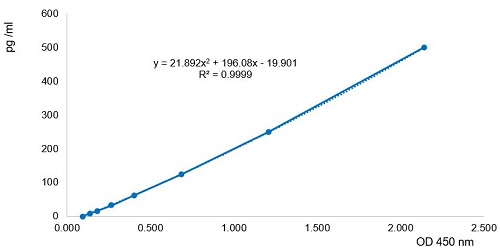
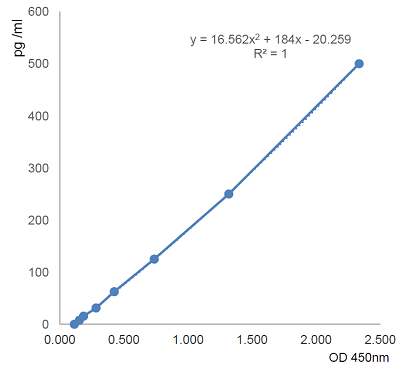
- Assay Detection Range7.8 to 500pg/ml
- Assay Sensitivity1.8pg/ml
- CertificationResearch Use Only
- Protein IDQ08830
- Protein NameFibrinogen-like protein 1
- Scientific DescriptionELISA Kit. Assay Type: Sandwich. Detection Type: Colorimetric. Sample Type: Cell Culture Supernatant,Plasma,Serum. Range: 7.8 to 500pg/ml. Sensitivity: 1.8pg/ml. FGL1 (Fibrinogen-like protein 1; also called Hepatocyte-derived fibrinogen-related protein 1; HFREP-1 or Hepassocin) was initially identified as an overexpressed transcript in hepatocyte carcinoma and as a transcript enriched in regenerating liver. FGL1 is expressed at lower levels in brown and white adipose in the setting of liver injury. A low level expression of FGL1 is also observed in the pancreas. FGL1 is a 34 kDa protein structurally similar to Angiopoietin-like factors 2, 3, 4 and 6, which regulate lipid metabolism and energy utilization. It was proposed that FGL1 is a member of an emerging group of proteins having potential roles in liver metabolism and liver regeneration. Recently, FGL1 has also been shown to be upregulated in human cancers and FGL1 is a major functional ligand of LAG-3. FGL1 interacts with LAG-3 in an MHC-II-independent manner and this interaction involves the FGL1 fibrinogen-like domain and the LAG-3 D1-D2 domain. FGL1-LAG-3 interaction blockade promotes tumor immunity by stimulating T cell expansion and activation. FGL1 forms two disulfide-linked homodimers and also higher molecular weight homooligomers that bind to LAG-3 much better than the dimeric forms. This binding to LAG-3 inhibits T cell responses. FGL1 is normally released by the liver in low levels but at higher levels in certain cancer such as non-small cell lung carcinomas (NSCLC) . Upregulated FGL1 in human cancers is associated with a poor prognosis. - FGL1 (Fibrinogen-like protein 1; also called Hepatocyte-derived fibrinogen-related protein 1; HFREP-1 or Hepassocin) was initially identified as an overexpressed transcript in hepatocyte carcinoma and as a transcript enriched in regenerating liver. FGL1 is expressed at lower levels in brown and white adipose in the setting of liver injury. A low level expression of FGL1 is also observed in the pancreas. FGL1 is a 34 kDa protein structurally similar to Angiopoietin-like factors 2, 3, 4 and 6, which regulate lipid metabolism and energy utilization. It was proposed that FGL1 is a member of an emerging group of proteins having potential roles in liver metabolism and liver regeneration. Recently, FGL1 has also been shown to be upregulated in human cancers and FGL1 is a major functional ligand of LAG-3. FGL1 interacts with LAG-3 in an MHC-II-independent manner and this interaction involves the FGL1 fibrinogen-like domain and the LAG-3 D1-D2 domain. FGL1-LAG-3 interaction blockade promotes tumor immunity by stimulating T cell expansion and activation. FGL1 forms two disulfide-linked homodimers and also higher molecular weight homooligomers that bind to LAG-3 much better than the dimeric forms. This binding to LAG-3 inhibits T cell responses. FGL1 is normally released by the liver in low levels but at higher levels in certain cancer such as non-small cell lung carcinomas (NSCLC) . Upregulated FGL1 in human cancers is associated with a poor prognosis.
- ReactivityHuman
- Storage Instruction2°C to 8°C
- UNSPSC41116100
- SpeciesHuman