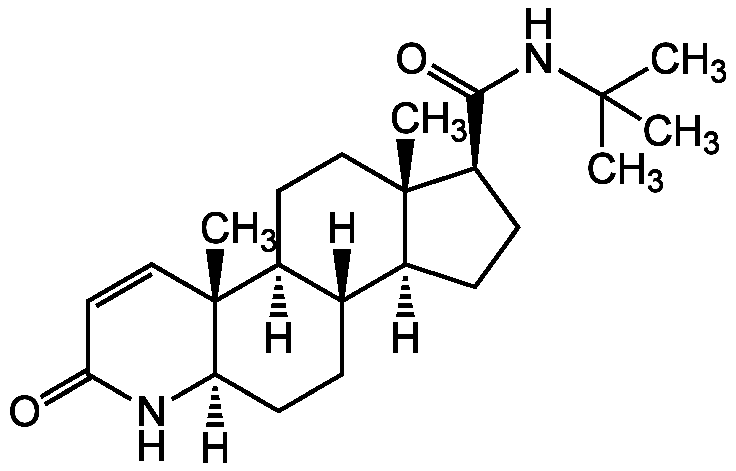
Chemical Structure
Finasteride [98319-26-7] [98319-26-7]
AG-CR1-3589
CAS Number98319-26-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight372.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameFinasteride [98319-26-7] [98319-26-7]
- Delivery Days Customer10
- CAS Number98319-26-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC23H36N2O2
- Molecular Weight372.5
- Scientific DescriptionChemical. CAS: 98319-26-7. Formula: C23H36N2O2. MW: 372.5. Potent, specific and competitive inhibitor of type II 5alpha-reductase (enzyme which converts testosterone to the more potent 5alpha-dihydrotestosterone). Anticancer compound. Apoptosis modulator. Enhances the action of GABA at GABA(A) receptors, which leads to neurological implications. Inhibit testosterone-induced type I procollagen and TGF-beta1 expression in human scalp dermal fibroblasts in a model of androgenic alopecia. Drug for the treatment of male androgenetic alopecia. Potential role in neuropsychiatric disorders. - Potent, specific and competitive inhibitor of type II 5alpha-reductase (enzyme which converts testosterone to the more potent 5alpha-dihydrotestosterone) [1, 3]. Anticancer compound [2, 6, 7]. Apoptosis modulator [2, 6]. Enhances the action of GABA at GABA(A) receptors, which leads to neurological implications [4]. Inhibit testosterone-induced type I procollagen and TGF-beta1 expression in human scalp dermal fibroblasts in a model of androgenic alopecia [5]. Drug for the treatment of male androgenetic alopecia [8]. Potential role in neuropsychiatric disorders [9]. Shown to reduce L-DOPA-induced dyskinesia in rodent models for Parkinsons disease [10].
- SMILES[H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])NC(=O)C=C[C@]12C
- Storage Instruction2°C to 8°C
- UNSPSC12352200


![Finasteride [98319-26-7] [98319-26-7]](https://www.targetmol.com/group3/M00/02/E5/CgoaEWY7O6KEFBceAAAAAM4vJf8930.png)