Chemical Structure
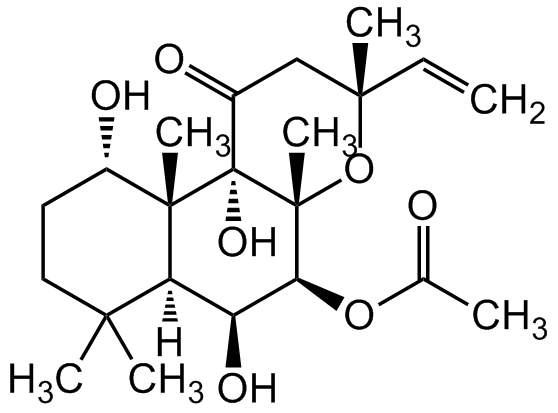
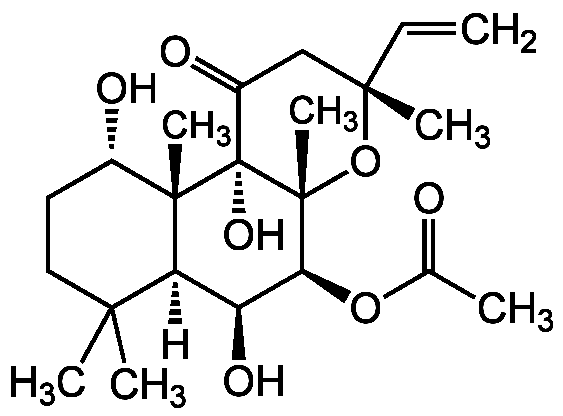
Forskolin [66428-89-5,66575-29-9] [66428-89-5]
AG-CN2-0089
CAS Number66428-89-5
Product group Chemicals
Estimated Purity>99%
Molecular Weight410.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameForskolin [66428-89-5,66575-29-9] [66428-89-5]
- Delivery Days Customer10
- CAS Number66428-89-5
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC22H34O7
- Molecular Weight410.5
- Scientific DescriptionChemical. CAS: 66428-89-5 and 66575-29-9. Formula: C22H34O7. MW: 410.5. Isolated from Coleus forskohlii. Potent, cell permeable adenylyl cyclase activator. Increases intracellular cAMP levels. Widely used tool to investigate cAMP as a second messenger. Inotropic and antihypertensive. Smooth muscle relaxant/vasodilator. Glucose transporter inhibitor. Platelet aggregation inhibitor. Stimulates lipolysis in fat cells. Non-competitive nicotinic acetylcholine receptors inhibitor. MAP kinase inhibitor. Upregulates mitochondrial uncoupling protein (UCP) mRNA levels in brown adipose tissue. Autophagy inhibitor. Hedgehog signaling inhibitor. Has antiglaucoma potential. Promotes neuronal differentiation of NSCs. - Potent, cell permeable adenylyl cyclase activator. Increases intracellular cAMP levels [1, 2, 6]. Widely used tool to investigate cAMP as a second messenger [15]. Inotropic and antihypertensive. Smooth muscle relaxant/vasodilator [3, 4, 6]. Glucose transporter inhibitor [4]. Platelet aggregation inhibitor [3, 10]. Stimulates lipolysis in fat cells [8]. Non-competitive nicotinic acetylcholine receptors inhibitor [9]. MAP kinase inhibitor [11]. Upregulates mitochondrial uncoupling protein (UCP) mRNA levels in brown adipose tissue [12]. Autophagy inhibitor [13]. Hedgehog signaling inhibitor [14]. Has antiglaucoma potential [16]. Promotes neuronal differentiation of NSCs [17]. Anti-neuroinflammatory and neuroprotective agent. Ameliorated Alzheimers disease symptoms, restoring impairment in nesting ability and sociability, and reducing neuroinflammation and amyloid beta deposition [18].
- SMILES[H][C@@]12[C@H](O)[C@H](OC(C)=O)[C@@]3(C)O[C@](C)(CC(=O)[C@]3(O)[C@@]1(C)[C@@H](O)CCC2(C)C)C=C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200