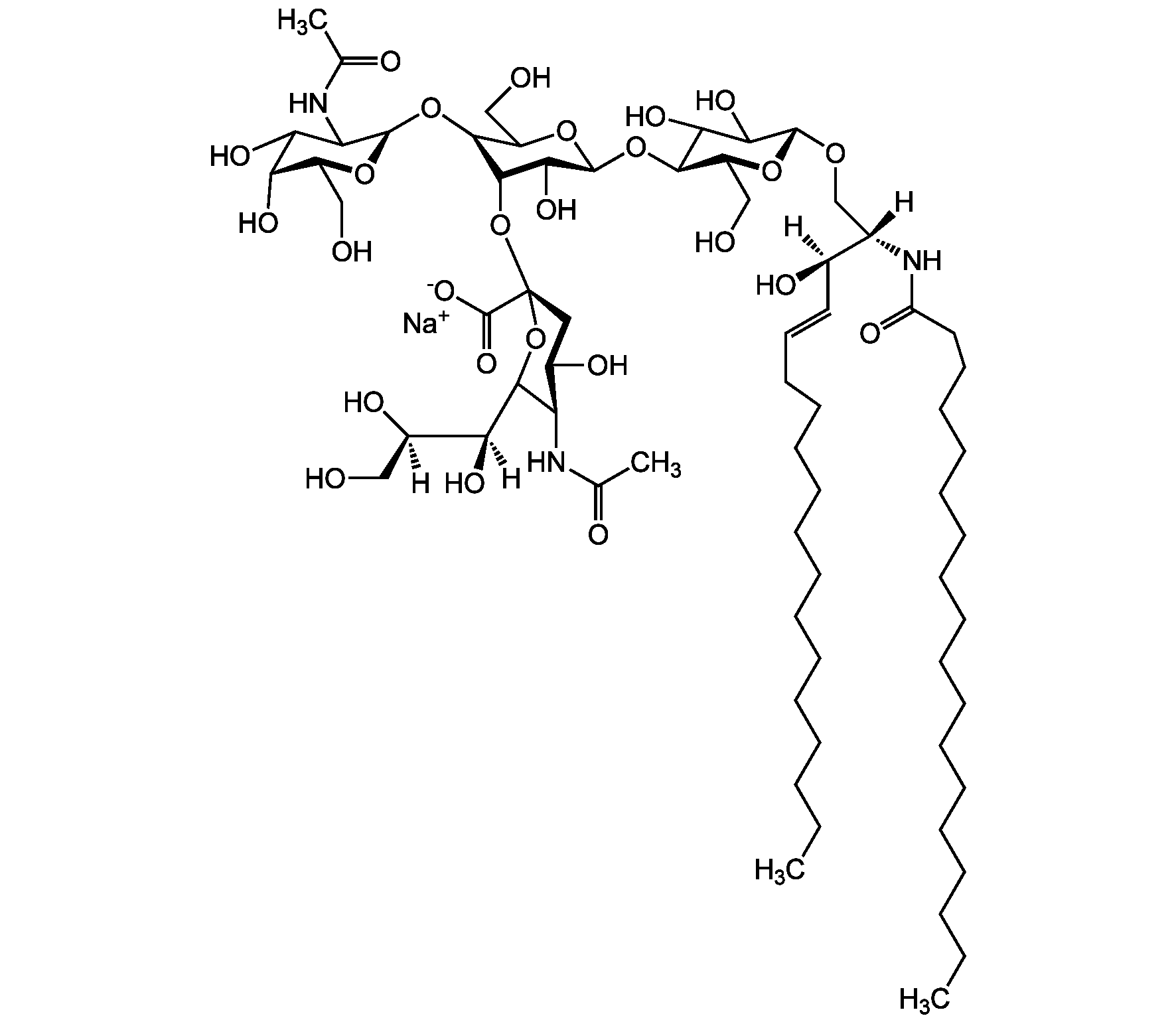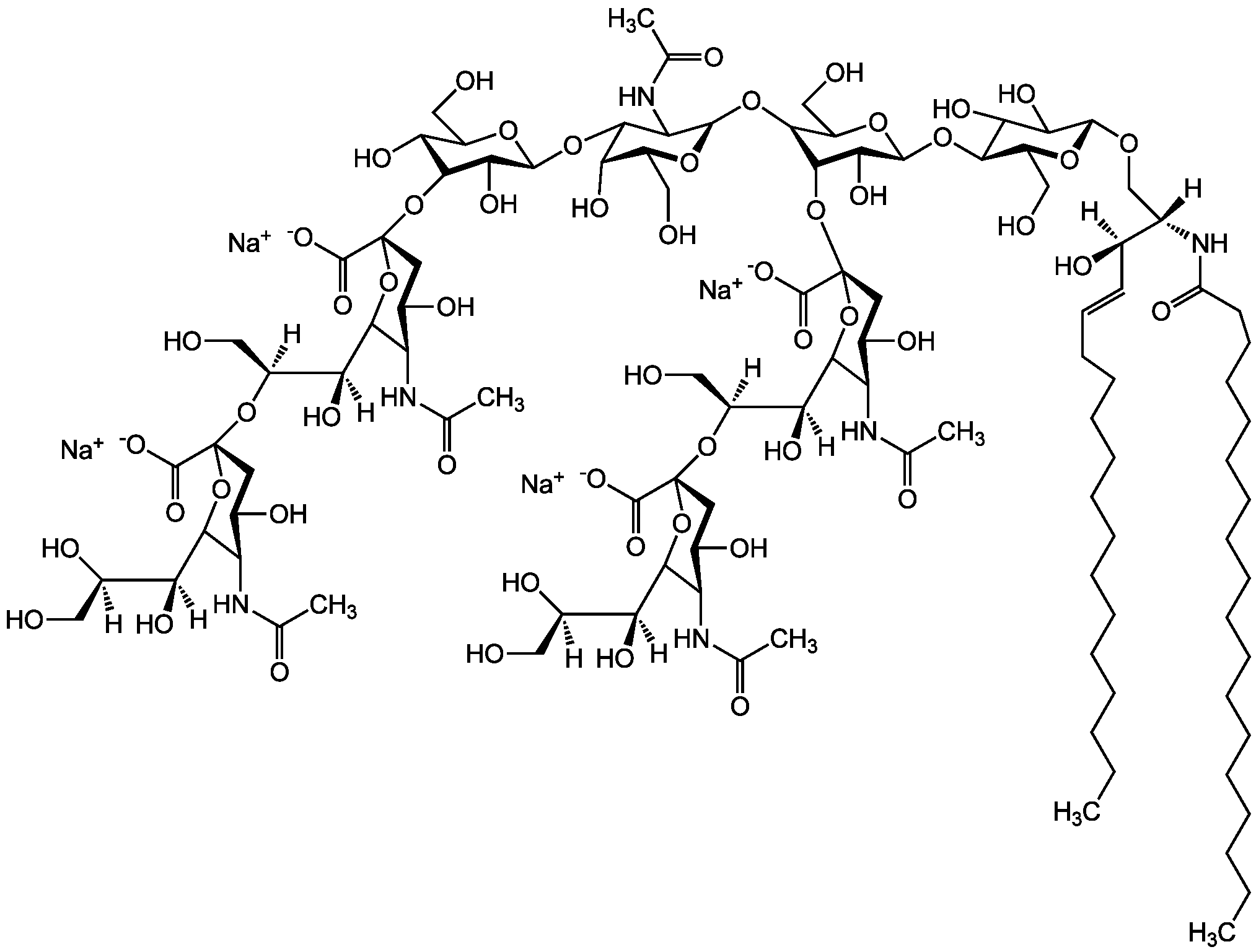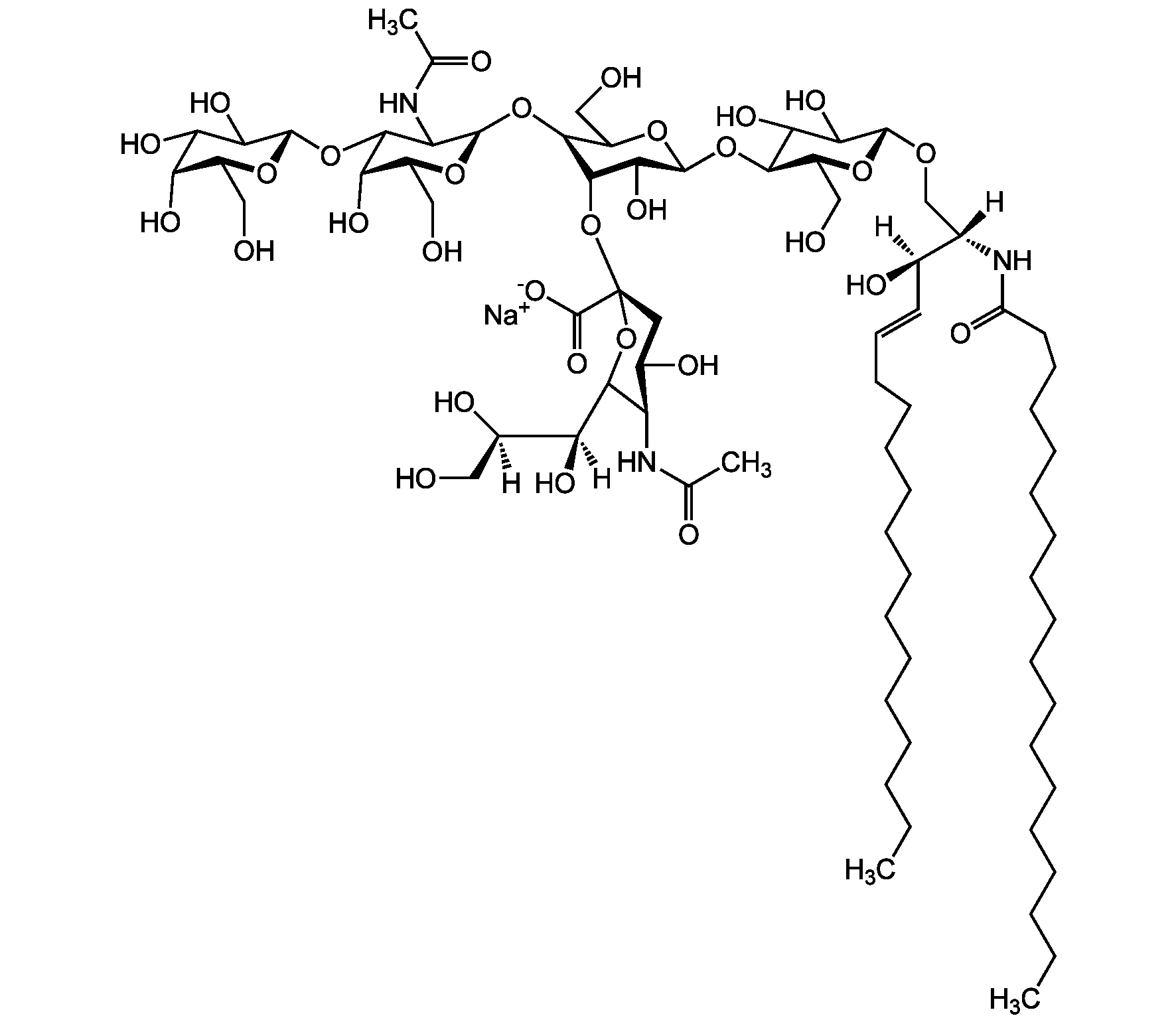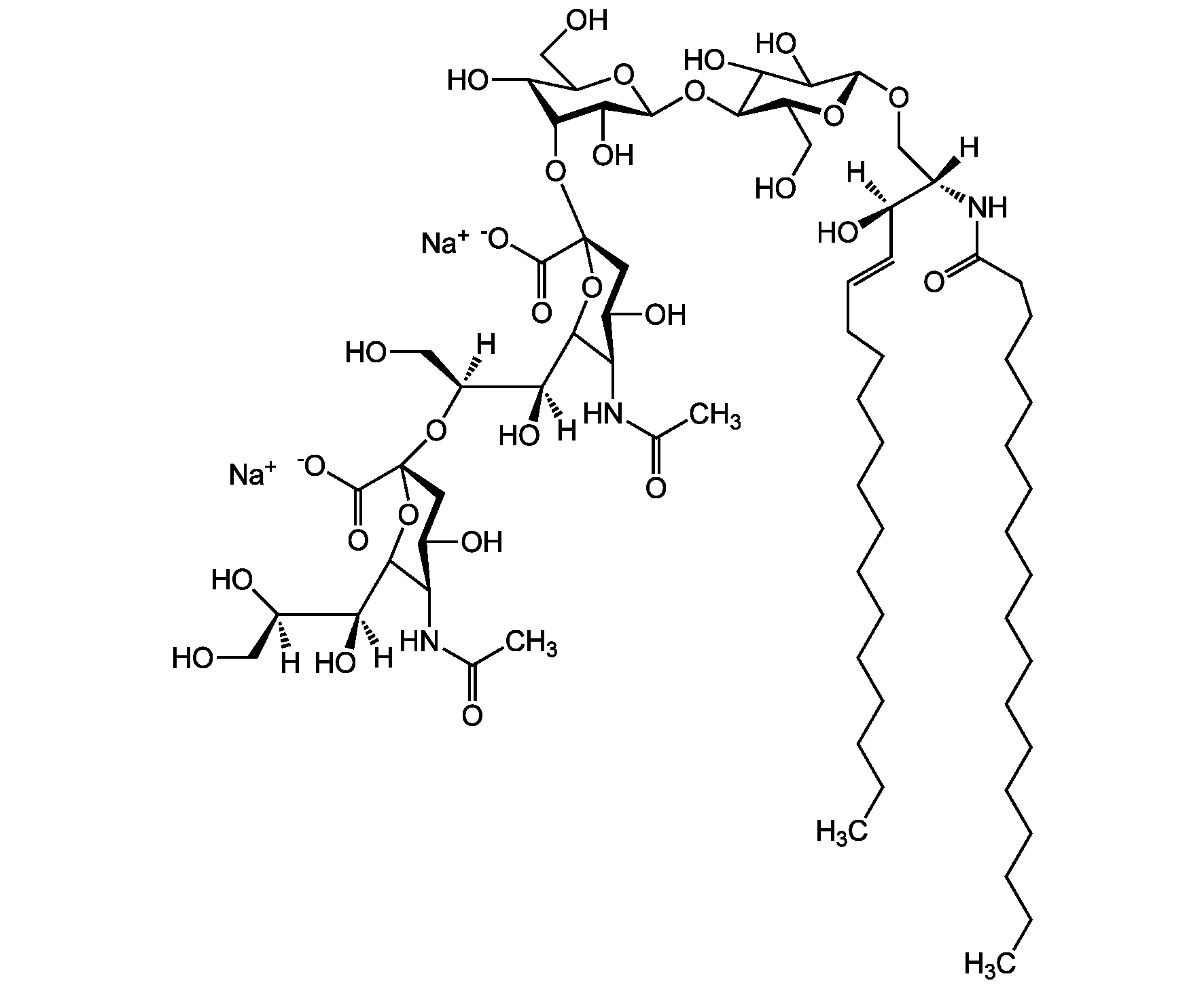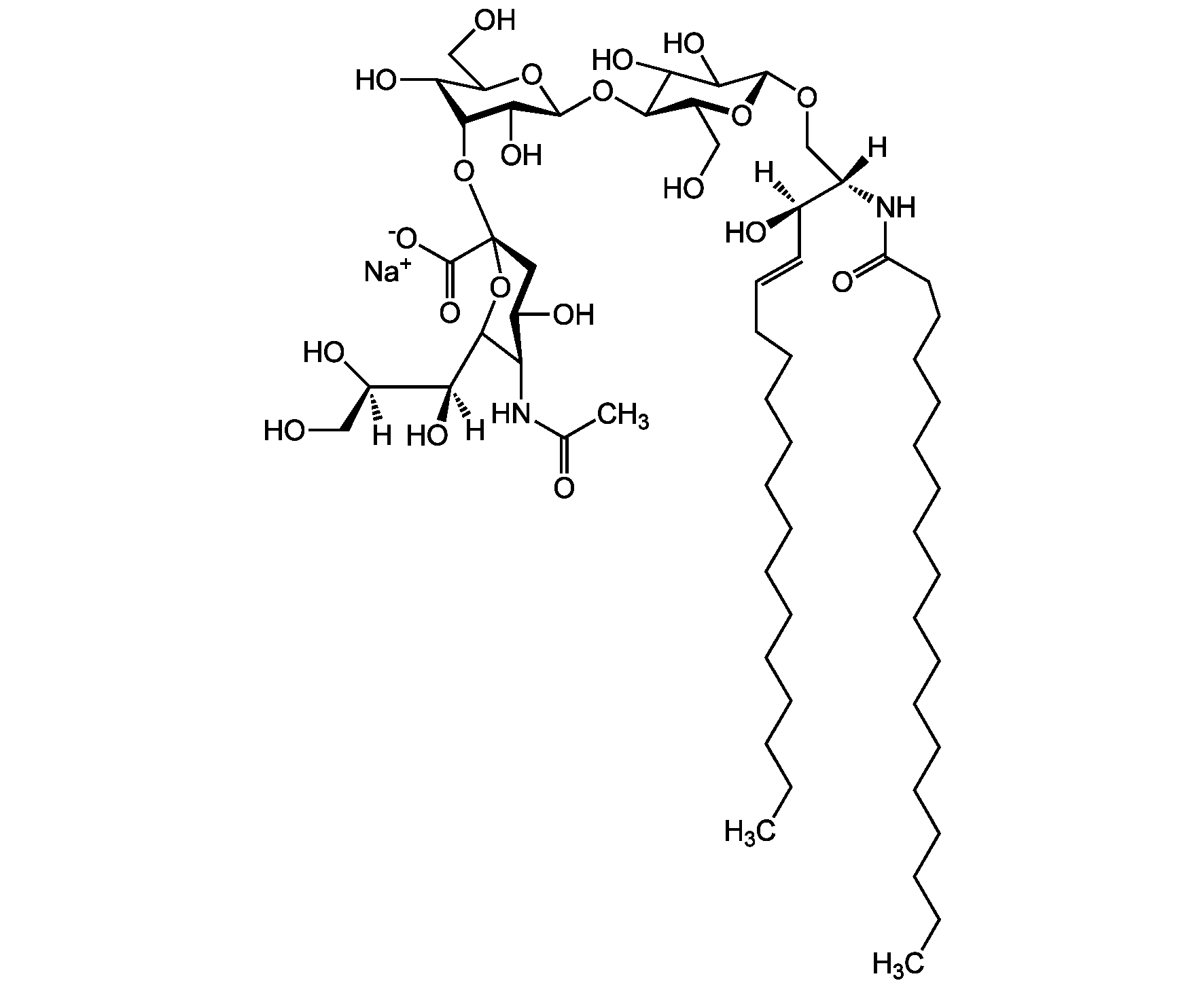
Chemical Structure
Ganglioside GM3 . sodium salt (bovine brain) [54827-14-4] [54827-14-4]
AG-CN2-9002
CAS Number54827-14-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight1180.5 . 23.0 (calculated on sphingosineC18:1 and stearic acid)
Overview
- SupplierAdipoGen Life Sciences
- Product NameGanglioside GM3 . sodium salt (bovine brain) [54827-14-4] [54827-14-4]
- Delivery Days Customer10
- CAS Number54827-14-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC59H107N2O21 . Na
- Molecular Weight1180.5 . 23.0 (calculated on sphingosineC18:1 and stearic acid)
- Scientific DescriptionChemical. CAS: 54827-14-4. Formula: C59H107N2O21 . Na. MW: 1180.5 . 23.0 (calculated on sphingosineC18:1 and stearic acid). Isolated from bovine brain. Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GM3 is the most abundant ganglioside in mammals. It is involved in several tumor processes by interacting with receptors. It inhibits epidermal cell growth and blocks insulin receptor activity. - Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GM3 is the most abundant ganglioside in mammals. It is involved in several tumor processes by interacting with receptors. It inhibits epidermal cell growth and blocks insulin receptor activity. Exogenous GM3 ganglioside inhibits atherosclerosis.
- SMILES[Na+].[H][C@@](O)(CO)[C@]([H])(O)C1O[C@@](CC(O)[C@H]1NC(C)=O)(O[C@H]1[C@H](O)C(CO)O[C@@H](O[C@H]2C(O)C(O)[C@H](OC[C@]([H])(NC(=O)CCCCCCCCCCCCCCCCC)[C@]([H])(O)\C=C\CCCCCCCCCCCCC)O[C@H]2CO)C1O)C([O-])=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200