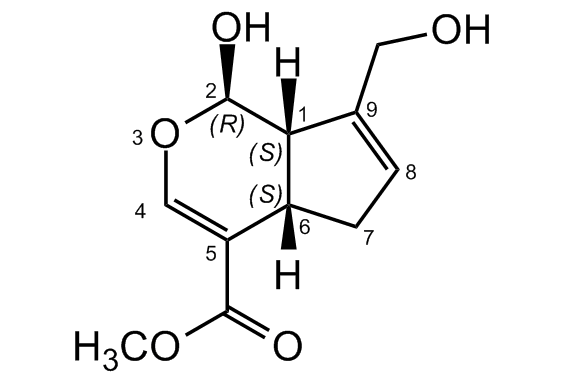
Chemical Structure
Genipin [6902-77-8] [6902-77-8]
AG-CN2-0481
CAS Number6902-77-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight226.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameGenipin [6902-77-8] [6902-77-8]
- Delivery Days Customer10
- CAS Number6902-77-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC11H14O5
- Molecular Weight226.2
- Scientific DescriptionCell permeable inhibitor of uncoupling protein 2 (UCP2). Increases glucose-stimulated insulin secretion, mitochondrial membrane potential and ATP levels in pancreatic island cells. Anticancer, antimetastatic and anti-angiogenic agent. Increases Bax/Bcl-2 ratio and induces PARP cleavage and caspase-3- and caspase-9-mediated apoptosis in non-small cell lung cancer (NSCLC) cells. Sensitizes multidrug-resistant cancer cells to cytotoxic agents through UCP2 inhibition. Suppresses the tumor promoting function of UCP2 in a cellular model of Warburg effect. Anti-inflammatory agent. Upregulates nNOS (neuronal nitric oxide synthase/NOS I) and decreases acute inflammation through inhibition of LPS-induced NF-kappaB activity. Inhibits toll-like receptor (TLR) signaling and the production of pro-inflammatory cytokines, preventing sepsis and increasing survival in animal models of infection. Modulates NLRP3 inflammasome activation and ATP- or H2O2-mediated IL-1beta release. Inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Antiviral compound. Chemical promoter of Epstein-Barr virus (EBV) lytic cycle activation and suppressor of EBV infection. Shown to have wound healing, neuroprotective, antidepressant, anti-osteoporotic and antioxidative activities. Protein, collagen, gelatin and chitosan natural cross-linking agent. 5,000-10,000 times less cytotoxic than any other crosslinking agents, with favorable properties of use in the range of pH 7.4-8.5 and temperatures between 25-45°C. Shown to be useful in the construction of novel drug delivery systems, as the raw material for gardenia blue pigment preparation and as the intermediate for alkaloid syntheses. Produces blue colored pigments by binding to amino acids. Simple, safe, sensitive and stable alternative for colorimetric determination of amino acids. Shown in forensic research to be a novel fingerprint reagent with colorimetric and fluorogenic activity. - Chemical. CAS: 6902-77-8. Formula: C11H14O5. MW: 226.2. Isolated from Gardenia jasminoides Ellis fruits. Cell permeable inhibitor of uncoupling protein 2 (UCP2). Increases glucose-stimulated insulin secretion, mitochondrial membrane potential and ATP levels in pancreatic island cells. Anticancer, antimetastatic and anti-angiogenic agent. Increases Bax/Bcl-2 ratio and induces PARP cleavage and caspase-3- and caspase-9-mediated apoptosis in non-small cell lung cancer (NSCLC) cells. Sensitizes multidrug-resistant cancer cells to cytotoxic agents through UCP2 inhibition. Suppresses the tumor promoting function of UCP2 in a cellular model of Warburg effect. Anti-inflammatory agent. Upregulates nNOS (neuronal nitric oxide synthase/NOS I) and decreases acute inflammation through inhibition of LPS-induced NF-kappaB activity. Inhibits toll-like receptor (TLR) signaling and the production of pro-inflammatory cytokines, preventing sepsis and increasing survival in animal models of infection. Modulates NLRP3 inflammasome activation and ATP- or H2O2-mediated IL-1beta release. Inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Antiviral compound. Chemical promoter of Epstein-Barr virus (EBV) lytic cycle activation and suppressor of EBV infection. Shown to have wound healing, neuroprotective, antidepressant, anti-osteoporotic and antioxidative activities. Protein, collagen, gelatin and chitosan natural cross-linking agent. 5,000-10,000 times less cytotoxic than any other crosslinking agents, with favorable properties of use in the range of pH 7.4-8.5 and temperatures between 25-45°C. Shown to be useful in the construction of novel drug delivery systems, as the raw material for gardenia blue pigment preparation and as the intermediate for alkaloid syntheses. Produces blue colored pigments by binding to amino acids. Simple, safe, sensitive and stable alternative for colorimetric determination of amino acids. Shown in forensic research to be a novel fingerprint reagent with colorimetric and fluorogenic activity.
- SMILES[H][C@]12CC=C(CO)[C@@]1([H])[C@H](O)OC=C2C(=O)OC
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200


![Genipin [6902-77-8] [6902-77-8]](https://www.targetmol.com/group3/M00/35/43/CgoaEGayGjqEO_2GAAAAAJgrIzw348.png)