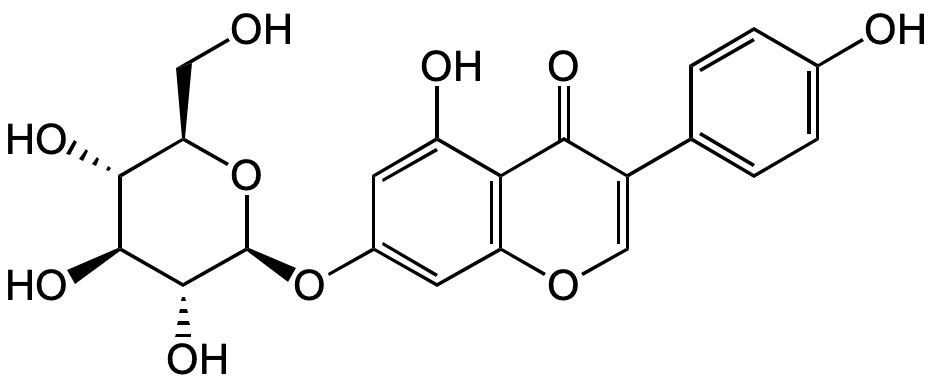
Chemical Structure
Genistin [529-59-9] [529-59-9]
CDX-G0191
CAS Number529-59-9
Product group Chemicals
Estimated Purity>97%
Molecular Weight432.38
Overview
- SupplierChemodex
- Product NameGenistin [529-59-9] [529-59-9]
- Delivery Days Customer10
- CAS Number529-59-9
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC21H20O10
- Molecular Weight432.38
- Scientific DescriptionChemical. CAS: 529-59-9. Formula: C21H20O10. MW: 432.38. Isolated from plant source. Genistin is a natural isoflavone glycoside isolated from legumes (e.g. soy). It is a selective inhibitor of mammalian terminal deoxyribonucleotidyl-transferase (TdT), with no measurable effect on mammalian or microbial DNA polymerases. Disrupts cell cycle and induces apoptosis in human ovarian cancer SK-OV-3 cells. Inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. It is a phytoestrogen, as it stimulates the growth of estrogen-dependent human breast cancer cells in vivo. Like other isoflavones, genistin promotes the proliferation of bone marrow stromal cells and osteoblasts and suppresses bone turnover. It also increases bone formation in collagen matrix in vivo. Could also be used as a negative control for the PTK inhibitor Genistein. - Genistin is a natural isoflavone glycoside isolated from legumes (e.g. soy). It is a selective inhibitor of mammalian terminal deoxyribonucleotidyl-transferase (TdT), with no measurable effect on mammalian or microbial DNA polymerases. Disrupts cell cycle and induces apoptosis in human ovarian cancer SK-OV-3 cells. Inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. It is a phytoestrogen, as it stimulates the growth of estrogen-dependent human breast cancer cells in vivo. Like other isoflavones, genistin promotes the proliferation of bone marrow stromal cells and osteoblasts and suppresses bone turnover. It also increases bone formation in collagen matrix in vivo. Could also be used as a negative control for the PTK inhibitor Genistein.
- SMILESOC[C@H]1O[C@@H](OC2=CC(OC=C(C3=CC=C(O)C=C3)C4=O)=C4C(O)=C2)[C@H](O)[C@@H](O)[C@@H]1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Genistin [529-59-9]](https://www.targetmol.com/group3/M00/37/E0/CgoaEWayUzeECy7KAAAAADVBHmQ124.png)