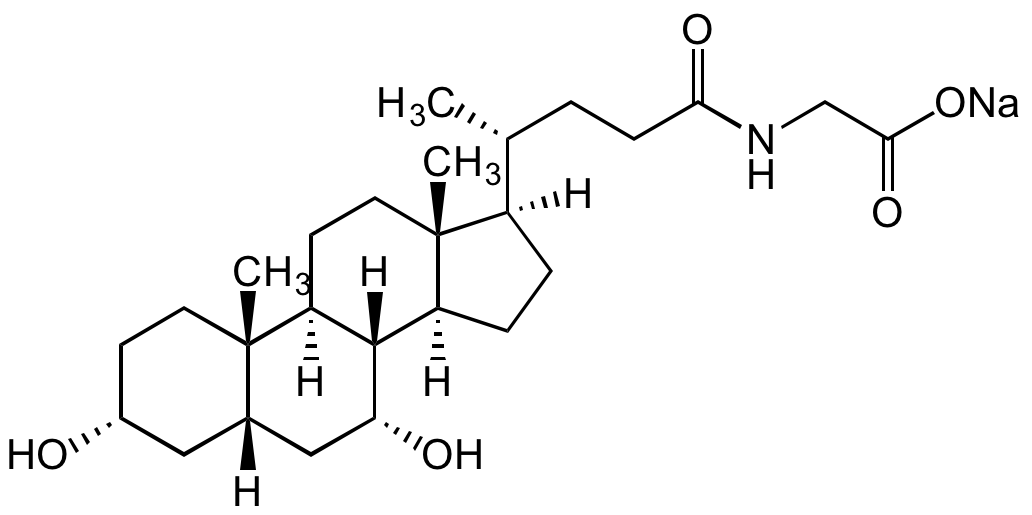
Chemical Structure
Glycochenodeoxycholic acid sodium salt [16564-43-5] [16564-43-5]
CDX-G0033
CAS Number16564-43-5
Product group Chemicals
Estimated Purity>97%
Molecular Weight471.61
Overview
- SupplierChemodex
- Product NameGlycochenodeoxycholic acid sodium salt [16564-43-5] [16564-43-5]
- Delivery Days Customer2
- CAS Number16564-43-5
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC26H42NNaO5
- Molecular Weight471.61
- Scientific DescriptionChemical. CAS: 16564-43-5. Formula: C26H42NNaO5. MW: 471.61. Synthetic. Glycochenodeoxycholic acid (GCDCA) is a bile salt formed in the liver that acts as a biosurfactant to solubilize lipids for absorption and is itself absorbed. GCDCA functions as a choleretic, increasing the volume of bile secreted from the liver, and a cholagogue, promoting the discharge of bile from the digestive system. GCDCA has been used to demonstrate a role for bile acids in promoting colorectal carcinogenesis and to inhibit calcium hydroxyapatite precipitation to study the pathogenesis of black pigment gallstones. This product has also been shown to induce apoptosis in isolated hepatocytes via modulation of PKC activity. This compound is used as an anionic detergent to solubilize lipids and as a chiral reagent. - Glycochenodeoxycholic acid (GCDCA) is a bile salt formed in the liver that acts as a biosurfactant to solubilize lipids for absorption and is itself absorbed. GCDCA functions as a choleretic, increasing the volume of bile secreted from the liver, and a cholagogue, promoting the discharge of bile from the digestive system. GCDCA has been used to demonstrate a role for bile acids in promoting colorectal carcinogenesis and to inhibit calcium hydroxyapatite precipitation to study the pathogenesis of black pigment gallstones. This product has also been shown to induce apoptosis in isolated hepatocytes via modulation of PKC activity. This compound is used as an anionic detergent to solubilize lipids and as a chiral reagent.
- SMILES[H][C@]1([C@@H](CCC(NCC(O[Na])=O)=O)C)CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@@]21C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Glycochenodeoxycholic acid sodium salt [16564-43-5] [16564-43-5]](https://www.targetmol.com/group3/M00/02/21/CgoaEGY7KbiESe39AAAAAG1HHfU741.png)