Chemical Structure
Harzianopyridone [126637-69-2] [126637-69-2]
AG-CN2-0149
CAS Number126637-69-2
Product group Chemicals
Estimated Purity>95%
Molecular Weight281.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameHarzianopyridone [126637-69-2] [126637-69-2]
- Delivery Days Customer10
- CAS Number126637-69-2
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
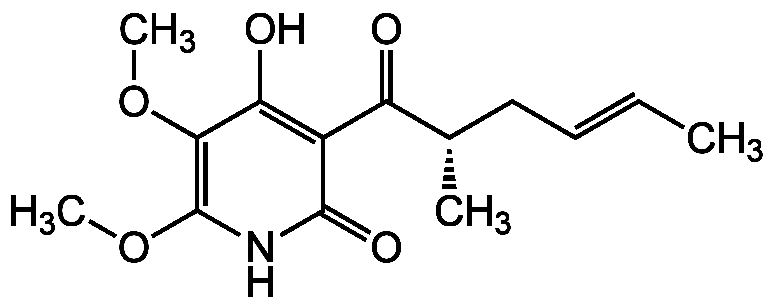
- Molecular FormulaC14H19NO5
- Molecular Weight281.3
- Scientific DescriptionAntibiotic [1]. Antifungal and antibacterial compound [1, 2, 6]. Specific mitochondrial complex II (succinate ubiquinone oxidoreductase; succinate dehydrogenase) inhibitor [3, 5]. Herbicidal activity [4]. Anthelmintic compound. Inhibits NADH-fumarate reductase activity of adult Ascaris suum mitochondria [3]. - Chemical. CAS: 126637-69-2. Formula: C14H19NO5. MW: 281.3. Isolated from Trichoderma harzianum FKI-1509. Antibiotic. Antifungal and antibacterial compound. Specific mitochondrial complex II (succinate ubiquinone oxidoreductase; succinate dehydrogenase) inhibitor. Herbicidal activity. Anthelmintic compound. Inhibits NADH-fumarate reductase activity of adult Ascaris suum mitochondria.
- SMILESCOC1=C(OC)C(O)=C(C(=O)[C@@H](C)C\C=C\C)C(=O)N1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
