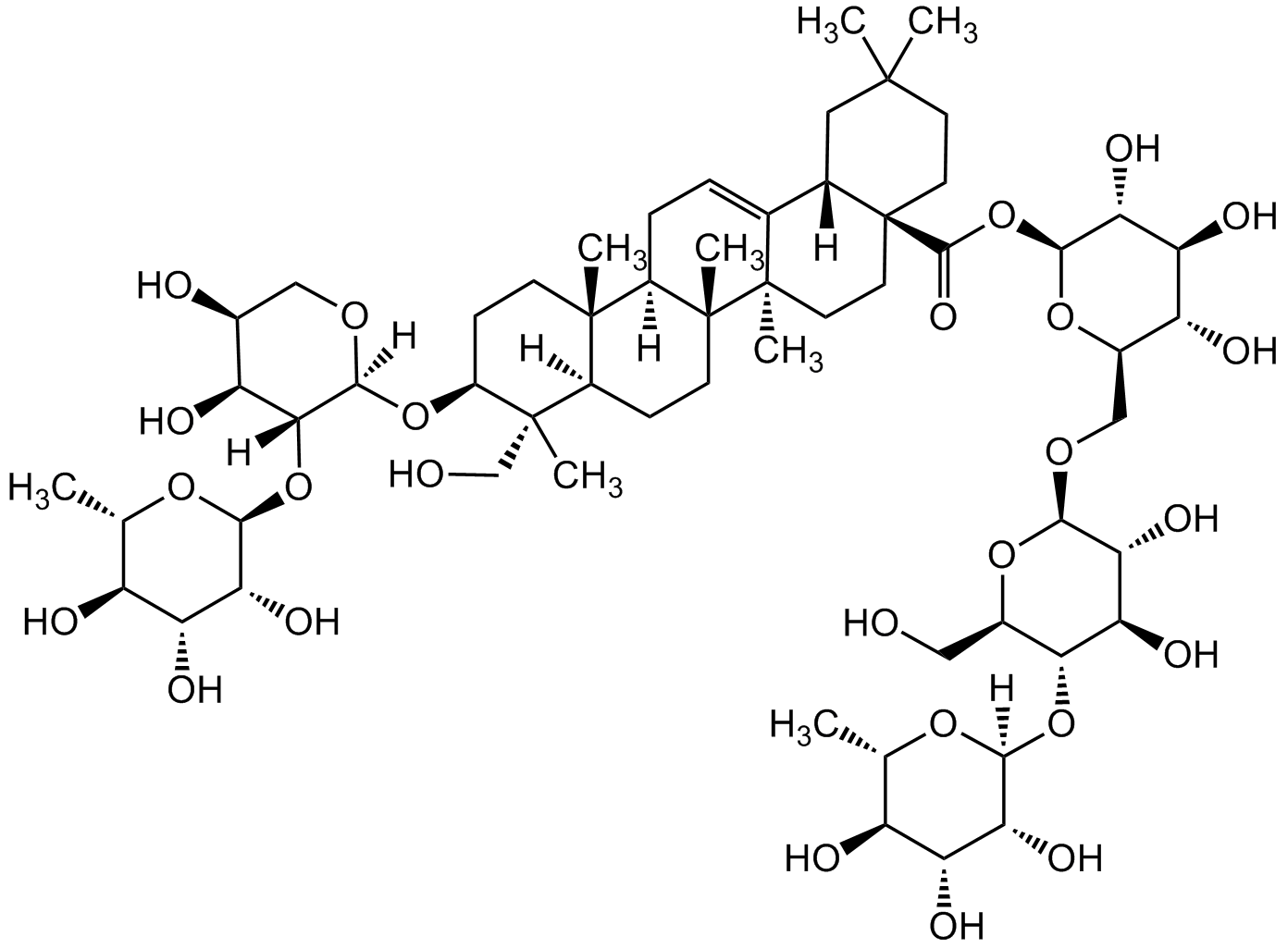
Chemical Structure
Hederacoside C
CDX-H0299
CAS Number14216-03-6
Product group Chemicals
Estimated Purity>98%
Molecular Weight1221.38
Overview
- SupplierChemodex
- Product NameHederacoside C
- Delivery Days Customer10
- CAS Number14216-03-6
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC59H96O26
- Molecular Weight1221.38
- Scientific DescriptionChemical. CAS: 14216-03-6. Formula: C59H96O26. MW: 1221.38. Hederacoside C is a major bioactive constituent isolated from Hedera helix L. It exhibits biological properties such as antibacterial, expectorant, bronchodilator, bronchospasmolytic and anti-inflammatroy effects. It is widely used in the treatment of respiratory disorders involving acute respiratory infections, chronic inflammatory bronchitis and productive coughs. It reduces TNF-alpha, IL-1beta, IL-6, COX-2, and nitric oxide synthase (NOS) levels in a concentration-dependent manner and IL-1 receptor-associated kinase-1 (IRAK1) activity. It reduces scopolamine-induced memory impairment. Hederacoside C markedly suppressed TLR2 and TLR4 expressions by attenuating the MAPKs (p38, ERK, JNK) and NF-kappa (p65 and IkappaBalpha) pathways followed by decreasing the phosphorylation of p38, ERK, JNK, p65, and IkappaBalpha. - Hederacoside C is a major bioactive constituent isolated from Hedera helix L. It exhibits biological properties such as antibacterial, expectorant, bronchodilator, bronchospasmolytic and anti-inflammatroy effects. It is widely used in the treatment of respiratory disorders involving acute respiratory infections, chronic inflammatory bronchitis and productive coughs. It reduces TNF-alpha, IL-1beta, IL-6, COX-2, and nitric oxide synthase (NOS) levels in a concentration-dependent manner and IL-1 receptor-associated kinase-1 (IRAK1) activity. It reduces scopolamine-induced memory impairment. Hederacoside C markedly suppressed TLR2 and TLR4 expressions by attenuating the MAPKs (p38, ERK, JNK) and NF-kappa (p65 and IkappaBalpha) pathways followed by decreasing the phosphorylation of p38, ERK, JNK, p65, and IkappaBalpha.
- SMILESO=C(O[C@H](O[C@@H]1CO[C@H](O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]2O[C@@](O[C@H]3C)([H])[C@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@@H](O)[C@@H]1O)[C@@]4(CC5)CC[C@]6(C)C([C@]4([H])CC5(C)C)=CC[C@@]7([H])[C@@]6(C)CC[C@]8([H])[C@]7(C)CC[C@H](O[C@@](OC[C@@H]9O)([H])[C@]([C@H]9O)([H])O[C@H](O[C@H]%10C)[C@H](O)[C@H](O)[C@H]%10O)[C@@]8(C)CO
- Storage Instruction2°C to 8°C,RT
- UN Number2811
- UNSPSC12352200

![Hederacoside C [14216-03-6]](https://www.targetmol.com/group3/M00/36/8A/CgoaEGayQCSEXOJ3AAAAABsLsc4016.png)
