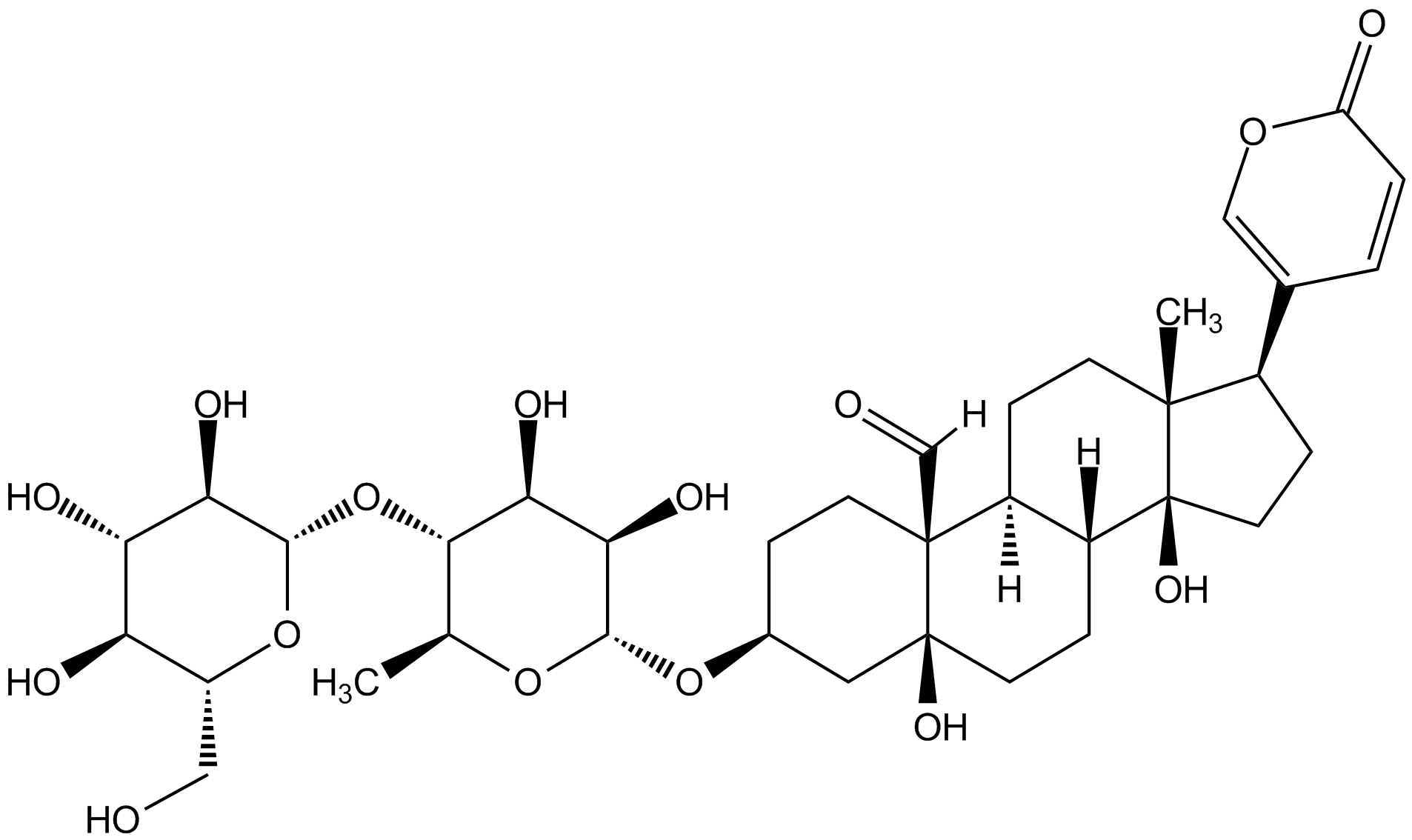
Chemical Structure
Hellebrin [13289-18-4] [13289-18-4]
AG-CN2-0475
CAS Number13289-18-4
Product group Chemicals
Estimated Purity>99%
Molecular Weight724.8
Overview
- SupplierAdipoGen Life Sciences
- Product NameHellebrin [13289-18-4] [13289-18-4]
- Delivery Days Customer10
- CAS Number13289-18-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC36H52O15
- Molecular Weight724.8
- Scientific DescriptionChemical. CAS: 13289-18-4. Formula: C36H52O15. MW: 724.8. Isolated from Helleborus purpurascens. Highly potent Na+/K+-ATPase inhibitor, blocking the active efflux of Na+ and reuptake of K+. Water soluble cardiotonic glycoside from the class of bufadienolides (with 2-pyrone ring). Inhibits cancer cell growth in vitro. Shown to induce caspase-dependent apoptosis, reducing MDR resistance and the rate of mitochondrial oxidative phosphorylation in cancer cells. Inotropic by increasing the intracellular Ca2+ concentration. Immunosuppressive and anti-inflammatory agent that inhibits allogeneic T cell activation with much higher potency than cortisol or cyclosporin A. - Highly potent Na+/K+-ATPase inhibitor, blocking the active efflux of Na+ and reuptake of K+. Water soluble cardiotonic glycoside from the class of bufadienolides (with 2-pyrone ring). Inhibits cancer cell growth in vitro. Shown to induce caspase-dependent apoptosis, reducing MDR resistance and the rate of mitochondrial oxidative phosphorylation in cancer cells. Inotropic by increasing the intracellular Ca2+ concentration. Immunosuppressive and anti-inflammatory agent that inhibits allogeneic T cell activation with much higher potency than cortisol or cyclosporin A. Anti-seizure activity in pentylenetetrazole (PTZ)-induced epileptical seizures.
- SMILES[H]C(=O)[C@]12CC[C@@H](C[C@@]1(O)CCC1C2CC[C@]2(C)[C@H](CC[C@]12O)C1=COC(=O)C=C1)O[C@@H]1OC(C)[C@H](O[C@@H]2OC(CO)[C@@H](O)[C@@H](O)C2O)[C@H](O)C1O
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200

![Hellebrin [13289-18-4] [13289-18-4]](https://www.targetmol.com/group3/M00/02/84/CgoaEWY7MCSENujtAAAAACtP3o4213.png)